Author: Zack Ulissi (Meta, CMU), with help from AI coding agents / LLMs
Original paper: Bjarne Kreitz et al. JPCC (2021)
Overview¶
This tutorial demonstrates how to use the Universal Model for Atoms (UMA) machine learning potential to perform comprehensive catalyst surface analysis. We replicate key computational workflows from “Microkinetic Modeling of CO₂ Desorption from Supported Multifaceted Ni Catalysts” by Bjarne Kreitz (now faculty at Georgia Tech!), showing how ML potentials can accelerate computational catalysis research.
Installation and Setup¶
This tutorial uses a number of helpful open source packages:
ase- Atomic Simulation Environmentfairchem- FAIR Chemistry ML potentials (formerly OCP)pymatgen- Materials analysismatplotlib- Visualizationnumpy- Numerical computingtorch-dftd- Dispersion corrections among many others!
Huggingface setups¶
You need to get a HuggingFace account and request access to the UMA models.
You need a Huggingface account, request access to https://
Permissions: Read access to contents of all public gated repos you can access
Then, add the token as an environment variable using huggingface-cli login:
# Enter token via huggingface-cli
! huggingface-cli loginor you can set the token via HF_TOKEN variable:
# Set token via env variable
import os
os.environ["HF_TOKEN"] = "MYTOKEN"FAIR Chemistry (UMA) installation¶
It may be enough to use pip install fairchem-core. This gets you the latest version on PyPi (https://
Here we install some sub-packages. This can take 2-5 minutes to run.
! pip install fairchem-core[docs] fairchem-data-oc fairchem-applications-cattsunami x3dase# Check that packages are installed
!pip list | grep fairchemfairchem-applications-cattsunami 1.1.2.dev182+gbafec1089
fairchem-core 2.14.1.dev13+gbafec1089
fairchem-data-oc 1.0.3.dev182+gbafec1089
fairchem-data-omat 0.2.1.dev87+gbafec1089
import fairchem.core
fairchem.core.__version__'2.14.1.dev13+gbafec1089'Package imports¶
First, let’s import all necessary libraries and initialize the UMA-S-1P1 predictor:
from pathlib import Path
import ase.io
import matplotlib.pyplot as plt
import numpy as np
from ase import Atoms
from ase.build import bulk
from ase.constraints import FixBondLengths
from ase.io import write
from ase.mep import interpolate
from ase.mep.dyneb import DyNEB
from ase.optimize import FIRE, LBFGS
from ase.vibrations import Vibrations
from ase.visualize import view
from fairchem.core import FAIRChemCalculator, pretrained_mlip
from fairchem.data.oc.core import (
Adsorbate,
AdsorbateSlabConfig,
Bulk,
MultipleAdsorbateSlabConfig,
Slab,
)
from pymatgen.analysis.wulff import WulffShape
from pymatgen.core import Lattice, Structure
from pymatgen.core.surface import SlabGenerator
from pymatgen.io.ase import AseAtomsAdaptor
from torch_dftd.torch_dftd3_calculator import TorchDFTD3Calculator
# Set up output directory structure
output_dir = Path("ni_tutorial_results")
output_dir.mkdir(exist_ok=True)
# Create subdirectories for each part
part_dirs = {
"part1": "part1-bulk-optimization",
"part2": "part2-surface-energies",
"part3": "part3-wulff-construction",
"part4": "part4-h-adsorption",
"part5": "part5-coverage-dependence",
"part6": "part6-co-dissociation",
}
for key, dirname in part_dirs.items():
(output_dir / dirname).mkdir(exist_ok=True)
# Create subdirectories for different facets in part2
for facet in ["111", "100", "110", "211"]:
(output_dir / part_dirs["part2"] / f"ni{facet}").mkdir(exist_ok=True)
# Initialize the UMA-S-1P1 predictor
print("\nLoading UMA-S-1P1 model...")
predictor = pretrained_mlip.get_predict_unit("uma-s-1p1")
print("✓ Model loaded successfully!")
Loading UMA-S-1P1 model...
WARNING:root:device was not explicitly set, using device='cuda'.
WARNING:root:If 'dataset_list' is provided in the config, the code assumes that each dataset maps to itself. Please use 'dataset_mapping' as 'dataset_list' is deprecated and will be removed in the future.
✓ Model loaded successfully!
It is somewhat time consuming to run this. We’re going to use a small number of bulks for the testing of this documentation, but otherwise run all of the results for the actual documentation.
import os
fast_docs = os.environ.get("FAST_DOCS", "false").lower() == "true"
if fast_docs:
num_sites = 2
relaxation_steps = 20
else:
num_sites = 5
relaxation_steps = 300Part 1: Bulk Crystal Optimization¶
Introduction¶
Before studying surfaces, we need to determine the equilibrium lattice constant of bulk Ni. This is crucial because surface energies and adsorbate binding depend strongly on the underlying lattice parameter.
Theory¶
For FCC metals like Ni, the lattice constant a defines the unit cell size. The experimental value for Ni is a = 3.524 Å at room temperature. We’ll optimize both atomic positions and the cell volume to find the ML potential’s equilibrium structure.
# Create initial FCC Ni structure
a_initial = 3.52 # Å, close to experimental
ni_bulk = bulk("Ni", "fcc", a=a_initial, cubic=True)
print(f"Initial lattice constant: {a_initial:.2f} Å")
print(f"Number of atoms: {len(ni_bulk)}")
# Set up calculator for bulk optimization
calc = FAIRChemCalculator(predictor, task_name="omat")
ni_bulk.calc = calc
# Use ExpCellFilter to allow cell relaxation
from ase.filters import ExpCellFilter
ecf = ExpCellFilter(ni_bulk)
# Optimize with LBFGS
opt = LBFGS(
ecf,
trajectory=str(output_dir / part_dirs["part1"] / "ni_bulk_opt.traj"),
logfile=str(output_dir / part_dirs["part1"] / "ni_bulk_opt.log"),
)
opt.run(fmax=0.05, steps=relaxation_steps)
# Extract results
cell = ni_bulk.get_cell()
a_optimized = cell[0, 0]
a_exp = 3.524 # Experimental value
error = abs(a_optimized - a_exp) / a_exp * 100
print(f"\n{'='*50}")
print(f"Experimental lattice constant: {a_exp:.2f} Å")
print(f"Optimized lattice constant: {a_optimized:.2f} Å")
print(f"Relative error: {error:.2f}%")
print(f"{'='*50}")
ase.io.write(str(output_dir / part_dirs["part1"] / "ni_bulk_relaxed.cif"), ni_bulk)
# Store results for later use
a_opt = a_optimizedInitial lattice constant: 3.52 Å
Number of atoms: 4
/tmp/ipykernel_9821/3959847705.py:15: DeprecationWarning: Use FrechetCellFilter for better convergence w.r.t. cell variables.
ecf = ExpCellFilter(ni_bulk)
==================================================
Experimental lattice constant: 3.52 Å
Optimized lattice constant: 3.52 Å
Relative error: 0.25%
==================================================
Part 2: Surface Energy Calculations¶
Introduction¶
Surface energy (γ) quantifies the thermodynamic cost of creating a surface. It determines surface stability, morphology, and catalytic activity. We’ll calculate γ for four low-index Ni facets: (111), (100), (110), and (211).
Theory¶
The surface energy is defined as:
where:
= total energy of the slab
= number of atoms in the slab
= bulk energy per atom
= surface area
Factor of 2 accounts for two surfaces (top and bottom)
Challenge: Direct calculation suffers from quantum size effects, and if you were doing DFT calculations small numerical errors in the simulation or from the K-point grid sampling can lead to small (but significant) errors in the bulk lattice energy.
Solution: It is fairly common when calculating surface energies to use the bulk energy from a bulk relaxation in the above equation. However, because DFT often has some small numerical noise in the predictions from k-point convergence, this might lead to the wrong surface energy. Instead, two more careful schemes are either:
Calculate the energy of a bulk structure oriented to each slab to maximize cancellation of small numerical errors or
Calculate the energy of multiple slabs at multiple thicknesses and extrapolate to zero thickness. The intercept will be the surface energy, and the slope will be a fitted bulk energy. A benefit of this approach is that it also forces us to check that we have a sufficiently thick slab for a well defined surface energy; if the fit is non-linear we need thicker slabs.
We’ll use the linear extrapolation method here as it’s more likely to work in future DFT studies if you use this code!
Step 1: Setup and Bulk Energy Reference¶
First, we’ll set up the calculation parameters and get the bulk energy reference:
# Calculate surface energies for all facets
facets = [(1, 1, 1), (1, 0, 0), (1, 1, 0), (2, 1, 1)]
surface_energies = {}
surface_energies_SI = {}
all_fit_data = {}
# Get bulk energy reference (only need to do this once)
E_bulk_total = ni_bulk.get_potential_energy()
N_bulk = len(ni_bulk)
E_bulk_per_atom = E_bulk_total / N_bulk
print(f"Bulk energy reference:")
print(f" Total energy: {E_bulk_total:.2f} eV")
print(f" Number of atoms: {N_bulk}")
print(f" Energy per atom: {E_bulk_per_atom:.6f} eV/atom")Bulk energy reference:
Total energy: -21.97 eV
Number of atoms: 4
Energy per atom: -5.491441 eV/atom
Step 2: Generate and Relax Slabs¶
Now we’ll loop through each facet, generating slabs at three different thicknesses:
# Convert bulk to pymatgen structure for slab generation
adaptor = AseAtomsAdaptor()
ni_structure = adaptor.get_structure(ni_bulk)
for facet in facets:
facet_str = "".join(map(str, facet))
print(f"\n{'='*60}")
print(f"Calculating Ni({facet_str}) surface energy")
print(f"{'='*60}")
# Calculate for three thicknesses
thicknesses = [4, 6, 8] # layers
n_atoms_list = []
energies_list = []
for n_layers in thicknesses:
print(f"\n Thickness: {n_layers} layers")
# Generate slab
slabgen = SlabGenerator(
ni_structure,
facet,
min_slab_size=n_layers * a_opt / np.sqrt(sum([h**2 for h in facet])),
min_vacuum_size=10.0,
center_slab=True,
)
pmg_slab = slabgen.get_slabs()[0]
slab = adaptor.get_atoms(pmg_slab)
slab.center(vacuum=10.0, axis=2)
print(f" Atoms: {len(slab)}")
# Relax slab (no constraints - both surfaces free)
calc = FAIRChemCalculator(predictor, task_name="omat")
slab.calc = calc
opt = LBFGS(slab, logfile=None)
opt.run(fmax=0.05, steps=relaxation_steps)
E_slab = slab.get_potential_energy()
n_atoms_list.append(len(slab))
energies_list.append(E_slab)
print(f" Energy: {E_slab:.2f} eV")
# Linear regression: E_slab = slope * N + intercept
coeffs = np.polyfit(n_atoms_list, energies_list, 1)
slope = coeffs[0]
intercept = coeffs[1]
# Extract surface energy from intercept
cell = slab.get_cell()
area = np.linalg.norm(np.cross(cell[0], cell[1]))
gamma = intercept / (2 * area) # eV/Ų
gamma_SI = gamma * 16.0218 # J/m²
print(f"\n Linear fit:")
print(f" Slope: {slope:.6f} eV/atom (cf. bulk {E_bulk_per_atom:.6f})")
print(f" Intercept: {intercept:.2f} eV")
print(f"\n Surface energy:")
print(f" γ = {gamma:.6f} eV/Ų = {gamma_SI:.2f} J/m²")
# Store results and fit data
surface_energies[facet] = gamma
surface_energies_SI[facet] = gamma_SI
all_fit_data[facet] = {
"n_atoms": n_atoms_list,
"energies": energies_list,
"slope": slope,
"intercept": intercept,
}
============================================================
Calculating Ni(111) surface energy
============================================================
Thickness: 4 layers
Atoms: 5
Energy: -26.18 eV
Thickness: 6 layers
Atoms: 7
Energy: -37.17 eV
Thickness: 8 layers
Atoms: 9
Energy: -48.15 eV
Linear fit:
Slope: -5.493045 eV/atom (cf. bulk -5.491441)
Intercept: 1.29 eV
Surface energy:
γ = 0.120198 eV/Ų = 1.93 J/m²
============================================================
Calculating Ni(100) surface energy
============================================================
Thickness: 4 layers
Atoms: 8
Energy: -42.16 eV
Thickness: 6 layers
Atoms: 12
Energy: -64.12 eV
Thickness: 8 layers
Atoms: 16
Energy: -86.09 eV
Linear fit:
Slope: -5.491520 eV/atom (cf. bulk -5.491441)
Intercept: 1.78 eV
Surface energy:
γ = 0.143731 eV/Ų = 2.30 J/m²
============================================================
Calculating Ni(110) surface energy
============================================================
Thickness: 4 layers
Atoms: 10
Energy: -52.35 eV
Thickness: 6 layers
Atoms: 14
Energy: -74.32 eV
Thickness: 8 layers
Atoms: 18
Energy: -96.29 eV
Linear fit:
Slope: -5.491905 eV/atom (cf. bulk -5.491441)
Intercept: 2.57 eV
Surface energy:
γ = 0.146934 eV/Ų = 2.35 J/m²
============================================================
Calculating Ni(211) surface energy
============================================================
Thickness: 4 layers
Atoms: 8
Energy: -39.69 eV
Thickness: 6 layers
Atoms: 12
Energy: -61.61 eV
Thickness: 8 layers
Atoms: 16
Energy: -83.58 eV
Linear fit:
Slope: -5.485733 eV/atom (cf. bulk -5.491441)
Intercept: 4.20 eV
Surface energy:
γ = 0.138877 eV/Ų = 2.23 J/m²
Step 3: Visualize Linear Fits¶
Let’s visualize the linear extrapolation for all four facets:
# Visualize linear fits for all facets
fig, axes = plt.subplots(2, 2, figsize=(12, 10))
axes = axes.flatten()
for idx, facet in enumerate(facets):
ax = axes[idx]
data = all_fit_data[facet]
# Plot data points
ax.scatter(
data["n_atoms"],
data["energies"],
s=100,
color="steelblue",
marker="o",
zorder=3,
label="Calculated",
)
# Plot fit line
n_range = np.linspace(min(data["n_atoms"]) - 5, max(data["n_atoms"]) + 5, 100)
E_fit = data["slope"] * n_range + data["intercept"]
ax.plot(
n_range,
E_fit,
"r--",
linewidth=2,
label=f'Fit: {data["slope"]:.2f}N + {data["intercept"]:.2f}',
)
# Formatting
facet_str = f"Ni({facet[0]}{facet[1]}{facet[2]})"
ax.set_xlabel("Number of Atoms", fontsize=11)
ax.set_ylabel("Slab Energy (eV)", fontsize=11)
ax.set_title(
f"{facet_str}: γ = {surface_energies_SI[facet]:.2f} J/m²",
fontsize=12,
fontweight="bold",
)
ax.legend(fontsize=9)
ax.grid(True, alpha=0.3)
plt.tight_layout()
plt.savefig(
str(output_dir / part_dirs["part2"] / "surface_energy_fits.png"),
dpi=300,
bbox_inches="tight",
)
plt.show()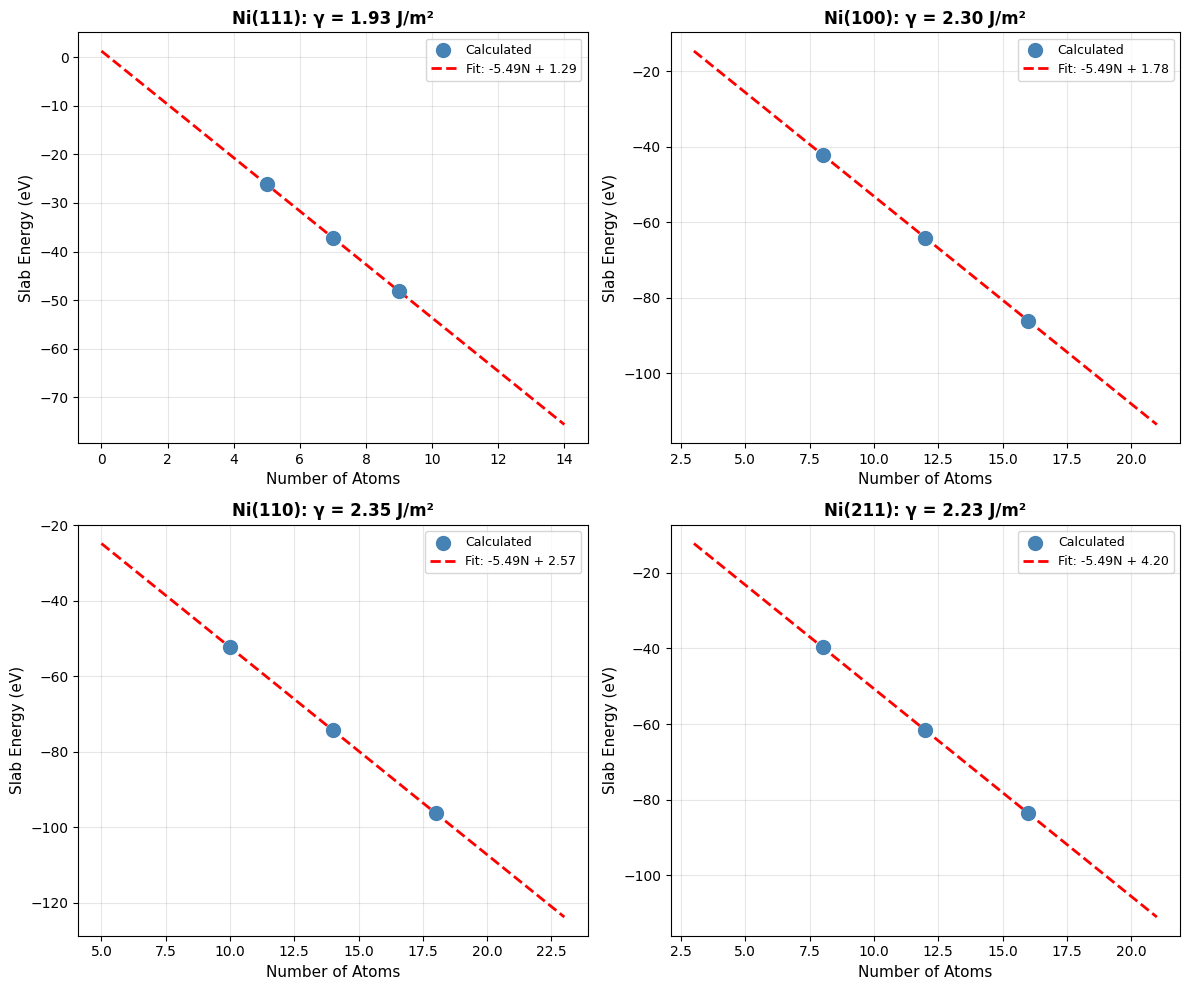
Step 4: Compare with Literature¶
Finally, let’s compare our calculated surface energies with DFT literature values:
print(f"\n{'='*70}")
print("Comparison with DFT Literature (Tran et al., 2016)")
print(f"{'='*70}")
lit_values = {
(1, 1, 1): 1.92,
(1, 0, 0): 2.21,
(1, 1, 0): 2.29,
(2, 1, 1): 2.24,
} # J/m²
for facet in facets:
facet_str = f"Ni({facet[0]}{facet[1]}{facet[2]})"
calc = surface_energies_SI[facet]
lit = lit_values[facet]
diff = abs(calc - lit) / lit * 100
print(f"{facet_str:<10} {calc:>8.2f} J/m² (Lit: {lit:.2f}, Δ={diff:.1f}%)")
======================================================================
Comparison with DFT Literature (Tran et al., 2016)
======================================================================
Ni(111) 1.93 J/m² (Lit: 1.92, Δ=0.3%)
Ni(100) 2.30 J/m² (Lit: 2.21, Δ=4.2%)
Ni(110) 2.35 J/m² (Lit: 2.29, Δ=2.8%)
Ni(211) 2.23 J/m² (Lit: 2.24, Δ=0.7%)
Explore on Your Own¶
Thickness convergence: Add 10 and 12 layer calculations. Is the linear fit still valid?
Constraint effects: Fix the bottom 2 layers during relaxation. How does this affect γ?
Vacuum size: Vary
min_vacuum_sizefrom 8 to 15 Å. When does γ converge?High-index facets: Try (311) or (331) surfaces. Are they more or less stable?
Alternative fitting: Use polynomial (degree 2) instead of linear fit. Does the intercept change?
Part 3: Wulff Construction¶
Introduction¶
The Wulff construction predicts the equilibrium shape of a crystalline particle by minimizing total surface energy. This determines the morphology of supported catalyst nanoparticles.
Theory¶
The Wulff theorem states that at equilibrium, the distance from the particle center to a facet is proportional to its surface energy:
Facets with lower surface energy have larger areas in the equilibrium shape.
Step 1: Prepare Surface Energies¶
We’ll use the surface energies calculated in Part 2 to construct the Wulff shape:
print("\nConstructing Wulff Shape")
print("=" * 50)
# Use optimized bulk structure
adaptor = AseAtomsAdaptor()
ni_structure = adaptor.get_structure(ni_bulk)
miller_list = list(surface_energies_SI.keys())
energy_list = [surface_energies_SI[m] for m in miller_list]
print(f"Using {len(miller_list)} facets:")
for miller, energy in zip(miller_list, energy_list):
print(f" {miller}: {energy:.2f} J/m²")
Constructing Wulff Shape
==================================================
Using 4 facets:
(1, 1, 1): 1.93 J/m²
(1, 0, 0): 2.30 J/m²
(1, 1, 0): 2.35 J/m²
(2, 1, 1): 2.23 J/m²
Step 2: Generate Wulff Construction¶
Now we create the Wulff shape and analyze its properties:
# Create Wulff shape
wulff = WulffShape(ni_structure.lattice, miller_list, energy_list)
# Print properties
print(f"\nWulff Shape Properties:")
print(f" Volume: {wulff.volume:.2f} ų")
print(f" Surface area: {wulff.surface_area:.2f} Ų")
print(f" Effective radius: {wulff.effective_radius:.2f} Å")
print(f" Weighted γ: {wulff.weighted_surface_energy:.2f} J/m²")
# Area fractions
print(f"\nFacet Area Fractions:")
area_frac = wulff.area_fraction_dict
for hkl, frac in sorted(area_frac.items(), key=lambda x: x[1], reverse=True):
print(f" {hkl}: {frac*100:.1f}%")
Wulff Shape Properties:
Volume: 44.64 ų
Surface area: 66.04 Ų
Effective radius: 2.20 Å
Weighted γ: 2.03 J/m²
Facet Area Fractions:
(1, 1, 1): 69.5%
(2, 1, 1): 17.3%
(1, 0, 0): 13.0%
(1, 1, 0): 0.3%
Step 3: Visualize and Compare¶
Let’s visualize the Wulff shape and compare with literature:
# Visualize
fig = wulff.get_plot()
plt.title("Wulff Construction: Ni Nanoparticle", fontsize=14)
plt.tight_layout()
plt.savefig(
str(output_dir / part_dirs["part3"] / "wulff_shape.png"),
dpi=300,
bbox_inches="tight",
)
plt.show()
# Compare with paper
print(f"\nComparison with Paper (Table 2):")
paper_fractions = {(1, 1, 1): 69.23, (1, 0, 0): 21.10, (1, 1, 0): 5.28, (2, 1, 1): 4.39}
for hkl in miller_list:
calc_frac = area_frac.get(hkl, 0) * 100
paper_frac = paper_fractions.get(hkl, 0)
print(f" {hkl}: {calc_frac:>6.1f}% (Paper: {paper_frac:.1f}%)")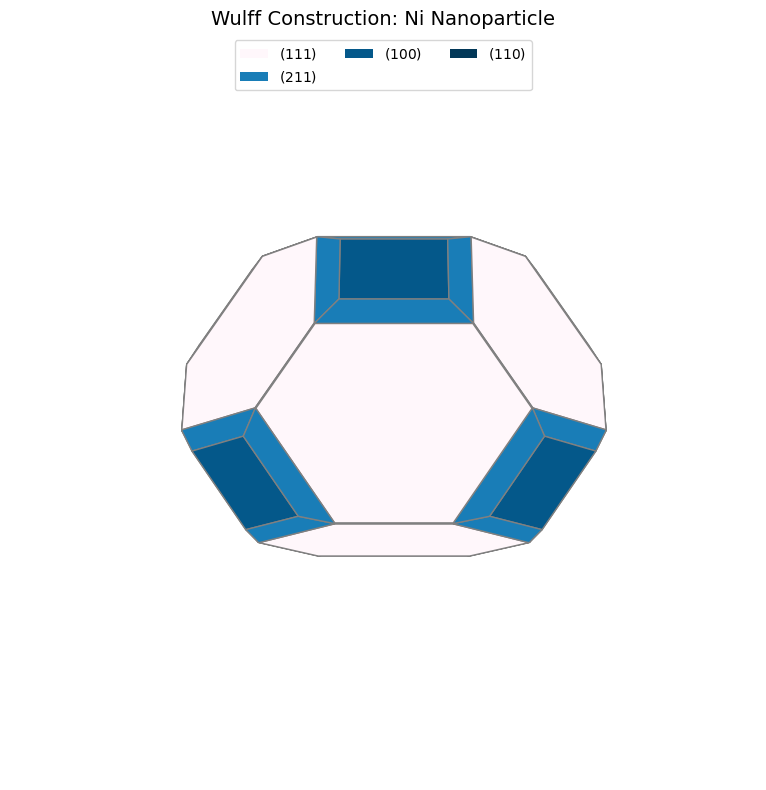
Comparison with Paper (Table 2):
(1, 1, 1): 69.5% (Paper: 69.2%)
(1, 0, 0): 13.0% (Paper: 21.1%)
(1, 1, 0): 0.3% (Paper: 5.3%)
(2, 1, 1): 17.3% (Paper: 4.4%)
Explore on Your Own¶
Particle size effects: How would including edge/corner energies modify the shape?
Anisotropic strain: Apply 2% compressive strain to the lattice. How does the shape change?
Temperature effects: Surface energies decrease with T. Estimate γ(T) and recompute Wulff shape.
Alloy nanoparticles: Replace some Ni with Cu or Au. How would segregation affect the shape?
Support effects: Some facets interact more strongly with supports. Model this by reducing their γ.
Part 4: H Adsorption Energy with ZPE Correction¶
Introduction¶
Hydrogen adsorption is a fundamental step in many catalytic reactions (hydrogenation, dehydrogenation, etc.). We’ll calculate the binding energy with vibrational zero-point energy (ZPE) corrections.
Theory¶
The adsorption energy is:
ZPE correction accounts for quantum vibrational effects:
The ZPE correction is calculated by analyzing the vibrational modes of the molecule/adsorbate.
Step 1: Setup and Relax Clean Slab¶
First, we create the Ni(111) surface and relax it:
# Create Ni(111) slab
ni_bulk_atoms = bulk("Ni", "fcc", a=a_opt, cubic=True)
ni_bulk_obj = Bulk(bulk_atoms=ni_bulk_atoms)
ni_slabs = Slab.from_bulk_get_specific_millers(
bulk=ni_bulk_obj, specific_millers=(1, 1, 1)
)
ni_slab = ni_slabs[0].atoms
print(f" Created {len(ni_slab)} atom slab")
# Set up calculators
calc = FAIRChemCalculator(predictor, task_name="oc20")
d3_calc = TorchDFTD3Calculator(device="cpu", damping="bj")
print(" Calculators initialized (ML + D3)") Created 96 atom slab
Calculators initialized (ML + D3)
Step 2: Relax Clean Slab¶
Relax the bare Ni(111) surface as our reference:
print("\n1. Relaxing clean Ni(111) slab...")
clean_slab = ni_slab.copy()
clean_slab.set_pbc([True, True, True])
clean_slab.calc = calc
opt = LBFGS(
clean_slab,
trajectory=str(output_dir / part_dirs["part4"] / "ni111_clean.traj"),
logfile=str(output_dir / part_dirs["part4"] / "ni111_clean.log"),
)
opt.run(fmax=0.05, steps=relaxation_steps)
E_clean_ml = clean_slab.get_potential_energy()
clean_slab.calc = d3_calc
E_clean_d3 = clean_slab.get_potential_energy()
E_clean = E_clean_ml + E_clean_d3
print(f" E(clean): {E_clean:.2f} eV (ML: {E_clean_ml:.2f}, D3: {E_clean_d3:.2f})")
# Save clean slab
ase.io.write(str(output_dir / part_dirs["part4"] / "ni111_clean.xyz"), clean_slab)
print(" ✓ Clean slab relaxed and saved")
1. Relaxing clean Ni(111) slab...
/home/runner/work/_tool/Python/3.12.12/x64/lib/python3.12/site-packages/torch_dftd/torch_dftd3_calculator.py:98: UserWarning: Creating a tensor from a list of numpy.ndarrays is extremely slow. Please consider converting the list to a single numpy.ndarray with numpy.array() before converting to a tensor. (Triggered internally at /pytorch/torch/csrc/utils/tensor_new.cpp:253.)
cell: Optional[Tensor] = torch.tensor(
E(clean): -487.46 eV (ML: -450.89, D3: -36.57)
✓ Clean slab relaxed and saved
Step 3: Generate H Adsorption Sites¶
Use heuristic placement to generate multiple candidate H adsorption sites:
print("\n2. Generating 5 H adsorption sites...")
ni_slab_for_ads = ni_slabs[0]
ni_slab_for_ads.atoms = clean_slab.copy()
adsorbate_h = Adsorbate(adsorbate_smiles_from_db="*H")
ads_slab_config = AdsorbateSlabConfig(
ni_slab_for_ads,
adsorbate_h,
mode="random_site_heuristic_placement",
num_sites=num_sites,
)
print(f" Generated {len(ads_slab_config.atoms_list)} initial configurations")
print(" These include fcc, hcp, bridge, and top sites")
2. Generating 5 H adsorption sites...
Generated 5 initial configurations
These include fcc, hcp, bridge, and top sites
Step 4: Relax All H Configurations¶
Relax each configuration and identify the most stable site:
print("\n3. Relaxing all H adsorption configurations...")
h_energies = []
h_configs = []
h_d3_energies = []
for idx, config in enumerate(ads_slab_config.atoms_list):
config_relaxed = config.copy()
config_relaxed.set_pbc([True, True, True])
config_relaxed.calc = calc
opt = LBFGS(
config_relaxed,
trajectory=str(output_dir / part_dirs["part4"] / f"h_site_{idx+1}.traj"),
logfile=str(output_dir / part_dirs["part4"] / f"h_site_{idx+1}.log"),
)
opt.run(fmax=0.05, steps=relaxation_steps)
E_ml = config_relaxed.get_potential_energy()
config_relaxed.calc = d3_calc
E_d3 = config_relaxed.get_potential_energy()
E_total = E_ml + E_d3
h_energies.append(E_total)
h_configs.append(config_relaxed)
h_d3_energies.append(E_d3)
print(f" Config {idx+1}: {E_total:.2f} eV (ML: {E_ml:.2f}, D3: {E_d3:.2f})")
# Save structure
ase.io.write(
str(output_dir / part_dirs["part4"] / f"h_site_{idx+1}.xyz"), config_relaxed
)
# Select best configuration
best_idx = np.argmin(h_energies)
slab_with_h = h_configs[best_idx]
E_with_h = h_energies[best_idx]
E_with_h_d3 = h_d3_energies[best_idx]
print(f"\n ✓ Best site: Config {best_idx+1}, E = {E_with_h:.2f} eV")
print(f" Energy spread: {max(h_energies) - min(h_energies):.2f} eV")
print(f" This spread indicates the importance of testing multiple sites!")
3. Relaxing all H adsorption configurations...
Config 1: -491.53 eV (ML: -454.88, D3: -36.65)
Config 2: -491.51 eV (ML: -454.86, D3: -36.65)
Config 3: -491.53 eV (ML: -454.88, D3: -36.65)
Config 4: -491.53 eV (ML: -454.88, D3: -36.65)
Config 5: -491.53 eV (ML: -454.88, D3: -36.65)
✓ Best site: Config 1, E = -491.53 eV
Energy spread: 0.02 eV
This spread indicates the importance of testing multiple sites!
Step 5: Calculate H₂ Reference Energy¶
We need the H₂ molecule energy as a reference:
print("\n4. Calculating H₂ reference energy...")
h2 = Atoms("H2", positions=[[0, 0, 0], [0, 0, 0.74]])
h2.center(vacuum=10.0)
h2.set_pbc([True, True, True])
h2.calc = calc
opt = LBFGS(
h2,
trajectory=str(output_dir / part_dirs["part4"] / "h2.traj"),
logfile=str(output_dir / part_dirs["part4"] / "h2.log"),
)
opt.run(fmax=0.05, steps=relaxation_steps)
E_h2_ml = h2.get_potential_energy()
h2.calc = d3_calc
E_h2_d3 = h2.get_potential_energy()
E_h2 = E_h2_ml + E_h2_d3
print(f" E(H₂): {E_h2:.2f} eV (ML: {E_h2_ml:.2f}, D3: {E_h2_d3:.2f})")
# Save H2 structure
ase.io.write(str(output_dir / part_dirs["part4"] / "h2_optimized.xyz"), h2)
4. Calculating H₂ reference energy...
E(H₂): -6.97 eV (ML: -6.97, D3: -0.00)
Step 6: Compute Adsorption Energy¶
Calculate the adsorption energy using the formula: E_ads = E(slab+H) - E(slab) - 0.5×E(H₂)
print(f"\n4. Computing Adsorption Energy:")
print(" E_ads = E(slab+H) - E(slab) - 0.5×E(H₂)")
E_ads = E_with_h - E_clean - 0.5 * E_h2
E_ads_no_d3 = (E_with_h - E_with_h_d3) - (E_clean - E_clean_d3) - 0.5 * (E_h2 - E_h2_d3)
print(f"\n Without D3: {E_ads_no_d3:.2f} eV")
print(f" With D3: {E_ads:.2f} eV")
print(f" D3 effect: {E_ads - E_ads_no_d3:.2f} eV")
print(f"\n → D3 corrections are negligible for H* (small, covalent bonding)")
4. Computing Adsorption Energy:
E_ads = E(slab+H) - E(slab) - 0.5×E(H₂)
Without D3: -0.51 eV
With D3: -0.59 eV
D3 effect: -0.08 eV
→ D3 corrections are negligible for H* (small, covalent bonding)
Step 7: Zero-Point Energy (ZPE) Corrections¶
Calculate vibrational frequencies to get ZPE corrections:
print("\n6. Computing ZPE corrections...")
print(" This accounts for quantum vibrational effects")
h_index = len(slab_with_h) - 1
slab_with_h.calc = calc
vib = Vibrations(slab_with_h, indices=[h_index], delta=0.02)
vib.run()
vib_energies = vib.get_energies()
zpe_ads = np.sum(vib_energies) / 2.0
h2.calc = calc
vib_h2 = Vibrations(h2, indices=[0, 1], delta=0.02)
vib_h2.run()
vib_energies_h2 = vib_h2.get_energies()
zpe_h2 = np.sum(vib_energies_h2) / 2.0
E_ads_zpe = E_ads + zpe_ads - 0.5 * zpe_h2
print(f" ZPE(H*): {zpe_ads:.2f} eV")
print(f" ZPE(H₂): {zpe_h2:.2f} eV")
print(f" E_ads(ZPE): {E_ads_zpe:.2f} eV")
# Visualize vibrational modes
print("\n Creating animations of vibrational modes...")
vib.write_mode(n=0)
ase.io.write("vib.0.gif", ase.io.read("vib.0.traj@:"), rotation=("-45x,0y,0z"))
vib.clean()
vib_h2.clean()
6. Computing ZPE corrections...
This accounts for quantum vibrational effects
ZPE(H*): 0.18+0.00j eV
ZPE(H₂): 0.41+0.00j eV
E_ads(ZPE): -0.61-0.00j eV
Creating animations of vibrational modes...
0
Step 8: Visualize and Compare Results¶
Visualize the best configuration and compare with literature:
print("\n7. Visualizing best H* configuration...")
view(slab_with_h, viewer='x3d')
7. Visualizing best H* configuration...
# 6. Compare with literature
print(f"\n{'='*60}")
print("Comparison with Literature:")
print(f"{'='*60}")
print("Table 4 (DFT): -0.60 eV (Ni(111), ref H₂)")
print(f"This work: {E_ads_zpe:.2f} eV")
print(f"Difference: {abs(E_ads_zpe - (-0.60)):.2f} eV")
============================================================
Comparison with Literature:
============================================================
Table 4 (DFT): -0.60 eV (Ni(111), ref H₂)
This work: -0.61-0.00j eV
Difference: 0.01 eV
Explore on Your Own¶
Site preference: Identify which site (fcc, hcp, bridge, top) the H prefers. Visualize with
view(atoms, viewer='x3d').Coverage effects: Place 2 H atoms on the slab. How does binding change with separation?
Different facets: Compare H adsorption on (100) and (110) surfaces. Which is strongest?
Subsurface H: Place H below the surface layer. Is it stable?
ZPE uncertainty: How sensitive is E_ads to the vibrational delta parameter (try 0.01, 0.03 Å)?
Part 5: Coverage-Dependent H Adsorption¶
Introduction¶
At higher coverages, adsorbate-adsorbate interactions become significant. We’ll study how H binding energy changes from dilute (1 atom) to saturated (full monolayer) coverage.
Theory¶
The differential adsorption energy at coverage θ is:
For many systems, this varies linearly:
where β quantifies lateral interactions (repulsive if β > 0).
Step 1: Setup Slab and Calculators¶
Create a larger Ni(111) slab to accommodate multiple adsorbates:
# Create large Ni(111) slab
ni_bulk_atoms = bulk("Ni", "fcc", a=a_opt, cubic=True)
ni_bulk_obj = Bulk(bulk_atoms=ni_bulk_atoms)
ni_slabs = Slab.from_bulk_get_specific_millers(
bulk=ni_bulk_obj, specific_millers=(1, 1, 1)
)
slab = ni_slabs[0].atoms.copy()
print(f" Created {len(slab)} atom slab")
# Set up calculators
base_calc = FAIRChemCalculator(predictor, task_name="oc20")
d3_calc = TorchDFTD3Calculator(device="cpu", damping="bj")
print(" ✓ Calculators initialized") Created 96 atom slab
✓ Calculators initialized
Step 2: Calculate Reference Energies¶
Get reference energies for clean surface and H₂:
Step 3: Set Up Coverage Study¶
Define the coverages we’ll test (from dilute to nearly 1 ML):
# Count surface sites
tags = slab.get_tags()
n_sites = np.sum(tags == 1)
print(f"\n3. Surface sites: {n_sites} (4×4 Ni(111))")
# Test coverages: 1 H, 0.25 ML, 0.5 ML, 0.75 ML, 1.0 ML
coverages_to_test = [1, 4, 8, 12, 16]
print(f"\n Will test coverages: {[f'{n/n_sites:.2f} ML' for n in coverages_to_test]}")
print(" This spans from dilute to nearly full monolayer")
coverages = []
adsorption_energies = []
3. Surface sites: 16 (4×4 Ni(111))
Will test coverages: ['0.06 ML', '0.25 ML', '0.50 ML', '0.75 ML', '1.00 ML']
This spans from dilute to nearly full monolayer
Step 4: Generate and Relax Configurations at Each Coverage¶
For each coverage, generate multiple configurations and find the lowest energy:
for n_h in coverages_to_test:
print(f"\n3. Coverage: {n_h} H ({n_h/n_sites:.2f} ML)")
# Generate configurations
ni_bulk_obj_h = Bulk(bulk_atoms=ni_bulk_atoms)
ni_slabs_h = Slab.from_bulk_get_specific_millers(
bulk=ni_bulk_obj_h, specific_millers=(1, 1, 1)
)
slab_for_ads = ni_slabs_h[0]
slab_for_ads.atoms = clean_slab.copy()
adsorbates_list = [Adsorbate(adsorbate_smiles_from_db="*H") for _ in range(n_h)]
try:
multi_ads_config = MultipleAdsorbateSlabConfig(
slab_for_ads, adsorbates_list, num_configurations=num_sites
)
except ValueError as e:
print(f" ⚠ Configuration generation failed: {e}")
continue
if len(multi_ads_config.atoms_list) == 0:
print(f" ⚠ No configurations generated")
continue
print(f" Generated {len(multi_ads_config.atoms_list)} configurations")
# Relax each and find best
config_energies = []
for idx, config in enumerate(multi_ads_config.atoms_list):
config_relaxed = config.copy()
config_relaxed.set_pbc([True, True, True])
config_relaxed.calc = base_calc
opt = LBFGS(config_relaxed, logfile=None)
opt.run(fmax=0.05, steps=relaxation_steps)
E_ml = config_relaxed.get_potential_energy()
config_relaxed.calc = d3_calc
E_d3 = config_relaxed.get_potential_energy()
E_total = E_ml + E_d3
config_energies.append(E_total)
print(f" Config {idx+1}: {E_total:.2f} eV")
best_idx = np.argmin(config_energies)
best_energy = config_energies[best_idx]
best_config = multi_ads_config.atoms_list[best_idx]
E_ads_per_h = (best_energy - E_clean - n_h * 0.5 * E_h2) / n_h
coverage = n_h / n_sites
coverages.append(coverage)
adsorption_energies.append(E_ads_per_h)
print(f" → E_ads/H: {E_ads_per_h:.2f} eV")
# Visualize best configuration at this coverage
print(f" Visualizing configuration with {n_h} H atoms...")
view(best_config, viewer='x3d')
print(f"\n✓ Completed coverage study: {len(coverages)} data points")
3. Coverage: 1 H (0.06 ML)
Generated 5 configurations
Config 1: -491.53 eV
Config 2: -491.51 eV
Config 3: -491.53 eV
Config 4: -491.53 eV
Config 5: -491.52 eV
→ E_ads/H: -0.59 eV
Visualizing configuration with 1 H atoms...
3. Coverage: 4 H (0.25 ML)
Generated 5 configurations
Config 1: -503.72 eV
Config 2: -503.70 eV
Config 3: -502.50 eV
Config 4: -503.43 eV
Config 5: -503.71 eV
→ E_ads/H: -0.58 eV
Visualizing configuration with 4 H atoms...
3. Coverage: 8 H (0.50 ML)
Generated 5 configurations
Config 1: -519.59 eV
Config 2: -519.63 eV
Config 3: -519.40 eV
Config 4: -519.57 eV
Config 5: -519.65 eV
→ E_ads/H: -0.54 eV
Visualizing configuration with 8 H atoms...
3. Coverage: 12 H (0.75 ML)
Generated 5 configurations
Config 1: -534.59 eV
Config 2: -534.80 eV
Config 3: -534.76 eV
Config 4: -535.29 eV
Config 5: -535.42 eV
→ E_ads/H: -0.51 eV
Visualizing configuration with 12 H atoms...
3. Coverage: 16 H (1.00 ML)
Generated 5 configurations
Config 1: -550.41 eV
Config 2: -549.28 eV
Config 3: -550.40 eV
Config 4: -550.87 eV
Config 5: -549.14 eV
→ E_ads/H: -0.48 eV
Visualizing configuration with 16 H atoms...
✓ Completed coverage study: 5 data points
Step 5: Perform Linear Fit¶
Fit E_ads vs coverage to extract the slope (lateral interaction strength):
print("\n4. Performing linear fit to coverage dependence...")
# Linear fit
from numpy.polynomial import Polynomial
p = Polynomial.fit(coverages, adsorption_energies, 1)
slope = p.coef[1]
intercept = p.coef[0]
print(f"\n{'='*60}")
print(f"Linear Fit: E_ads = {intercept:.2f} + {slope:.2f}θ (eV)")
print(f"Slope: {slope * 96.485:.1f} kJ/mol per ML")
print(f"Paper: 8.7 kJ/mol per ML")
print(f"{'='*60}")
4. Performing linear fit to coverage dependence...
============================================================
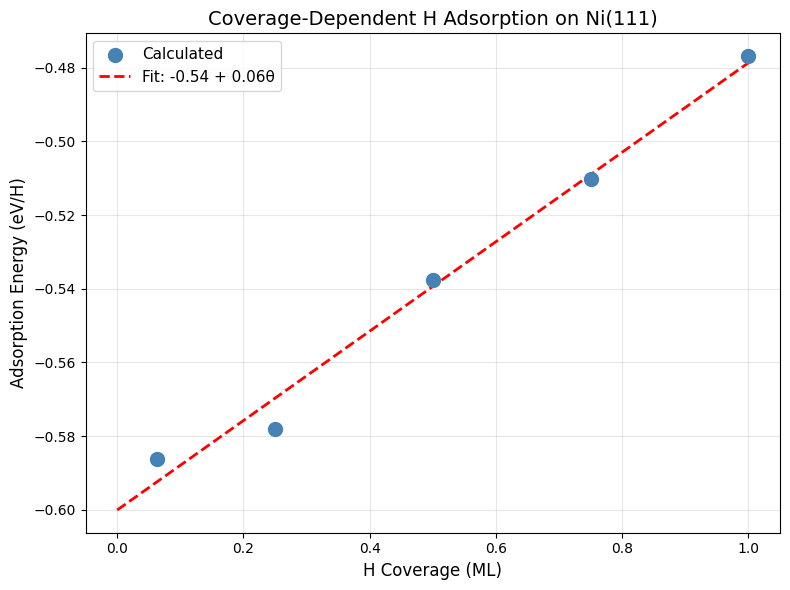
Linear Fit: E_ads = -0.54 + 0.06θ (eV)
Slope: 5.5 kJ/mol per ML
Paper: 8.7 kJ/mol per ML
============================================================
Step 6: Visualize Coverage Dependence¶
Create a plot showing how adsorption energy changes with coverage:
print("\n5. Plotting coverage dependence...")
# Plot
fig, ax = plt.subplots(figsize=(8, 6))
ax.scatter(
coverages,
adsorption_energies,
s=100,
marker="o",
label="Calculated",
zorder=3,
color="steelblue",
)
cov_fit = np.linspace(0, max(coverages), 100)
ads_fit = p(cov_fit)
ax.plot(
cov_fit, ads_fit, "r--", label=f"Fit: {intercept:.2f} + {slope:.2f}θ", linewidth=2
)
ax.set_xlabel("H Coverage (ML)", fontsize=12)
ax.set_ylabel("Adsorption Energy (eV/H)", fontsize=12)
ax.set_title("Coverage-Dependent H Adsorption on Ni(111)", fontsize=14)
ax.legend(fontsize=11)
ax.grid(True, alpha=0.3)
plt.tight_layout()
plt.savefig(str(output_dir / part_dirs["part5"] / "coverage_dependence.png"), dpi=300)
plt.show()
print("\n✓ Coverage dependence analysis complete!")
5. Plotting coverage dependence...
✓ Coverage dependence analysis complete!
Explore on Your Own¶
Non-linear behavior: Use polynomial (degree 2) fit. Is there curvature at high coverage?
Temperature effects: Estimate configurational entropy at each coverage. How does this affect free energy?
Pattern formation: Visualize the lowest-energy configuration at 0.5 ML. Are H atoms ordered?
Other adsorbates: Repeat for O or N. How do lateral interactions compare?
Phase diagrams: At what coverage do you expect phase separation (islands vs uniform)?
Part 6: CO Formation/Dissociation Thermochemistry and Barrier¶
Introduction¶
CO dissociation (CO* → C* + O*) is the rate-limiting step in many catalytic processes (Fischer-Tropsch, CO oxidation, etc.). We’ll calculate the reaction energy for C* + O* → CO* and the activation barriers in both directions using the nudged elastic band (NEB) method.
Theory¶
Forward Reaction: C* + O* → CO* + * (recombination)
Reverse Reaction: CO* + → C + O* (dissociation)
Thermochemistry:
Barrier: NEB finds the minimum energy path (MEP) and transition state:
Step 1: Setup Slab and Calculators¶
Initialize the Ni(111) surface and calculators:
# Create slab
ni_bulk_atoms = bulk("Ni", "fcc", a=a_opt, cubic=True)
ni_bulk_obj = Bulk(bulk_atoms=ni_bulk_atoms)
ni_slabs = Slab.from_bulk_get_specific_millers(
bulk=ni_bulk_obj, specific_millers=(1, 1, 1)
)
slab = ni_slabs[0].atoms
print(f" Created {len(slab)} atom slab")
base_calc = FAIRChemCalculator(predictor, task_name="oc20")
d3_calc = TorchDFTD3Calculator(device="cpu", damping="bj")
print(" \u2713 Calculators initialized") Created 96 atom slab
✓ Calculators initialized
Step 2: Generate and Relax Final State (CO*)¶
Find the most stable CO adsorption configuration (this is the product of C+O recombination):
print("\n1. Final State: CO* on Ni(111)")
print(" Generating CO adsorption configurations...")
ni_bulk_obj_co = Bulk(bulk_atoms=ni_bulk_atoms)
ni_slab_co = Slab.from_bulk_get_specific_millers(
bulk=ni_bulk_obj_co, specific_millers=(1, 1, 1)
)[0]
ni_slab_co.atoms = slab.copy()
adsorbate_co = Adsorbate(adsorbate_smiles_from_db="*CO")
multi_ads_config_co = MultipleAdsorbateSlabConfig(
ni_slab_co, [adsorbate_co], num_configurations=num_sites
)
print(f" Generated {len(multi_ads_config_co.atoms_list)} configurations")
# Relax and find best
co_energies = []
co_energies_ml = []
co_energies_d3 = []
co_configs = []
for idx, config in enumerate(multi_ads_config_co.atoms_list):
config_relaxed = config.copy()
config_relaxed.set_pbc([True, True, True])
config_relaxed.calc = base_calc
opt = LBFGS(config_relaxed, logfile=None)
opt.run(fmax=0.05, steps=relaxation_steps)
E_ml = config_relaxed.get_potential_energy()
config_relaxed.calc = d3_calc
E_d3 = config_relaxed.get_potential_energy()
E_total = E_ml + E_d3
co_energies.append(E_total)
co_energies_ml.append(E_ml)
co_energies_d3.append(E_d3)
co_configs.append(config_relaxed)
print(
f" Config {idx+1}: E_total = {E_total:.2f} eV (RPBE: {E_ml:.2f}, D3: {E_d3:.2f})"
)
best_co_idx = np.argmin(co_energies)
final_co = co_configs[best_co_idx]
E_final_co = co_energies[best_co_idx]
E_final_co_ml = co_energies_ml[best_co_idx]
E_final_co_d3 = co_energies_d3[best_co_idx]
print(f"\n → Best CO* (Config {best_co_idx+1}):")
print(f" RPBE: {E_final_co_ml:.2f} eV")
print(f" D3: {E_final_co_d3:.2f} eV")
print(f" Total: {E_final_co:.2f} eV")
# Save best CO state
ase.io.write(str(output_dir / part_dirs["part6"] / "co_final_best.traj"), final_co)
print(" ✓ Best CO* structure saved")
# Visualize best CO* structure
print("\n Visualizing best CO* structure...")
view(final_co, viewer='x3d')
1. Final State: CO* on Ni(111)
Generating CO adsorption configurations...
Generated 5 configurations
Config 1: E_total = -503.96 eV (RPBE: -467.14, D3: -36.82)
Config 2: E_total = -503.96 eV (RPBE: -467.14, D3: -36.82)
Config 3: E_total = -503.97 eV (RPBE: -467.14, D3: -36.82)
Config 4: E_total = -503.97 eV (RPBE: -467.14, D3: -36.82)
Config 5: E_total = -503.97 eV (RPBE: -467.14, D3: -36.82)
→ Best CO* (Config 5):
RPBE: -467.14 eV
D3: -36.82 eV
Total: -503.97 eV
✓ Best CO* structure saved
Visualizing best CO* structure...
Step 3: Generate and Relax Initial State (C* + O*)¶
Find the most stable configuration for dissociated C and O (reactants):
print("\n2. Initial State: C* + O* on Ni(111)")
print(" Generating C+O configurations...")
ni_bulk_obj_c_o = Bulk(bulk_atoms=ni_bulk_atoms)
ni_slab_c_o = Slab.from_bulk_get_specific_millers(
bulk=ni_bulk_obj_c_o, specific_millers=(1, 1, 1)
)[0]
adsorbate_c = Adsorbate(adsorbate_smiles_from_db="*C")
adsorbate_o = Adsorbate(adsorbate_smiles_from_db="*O")
multi_ads_config_c_o = MultipleAdsorbateSlabConfig(
ni_slab_c_o, [adsorbate_c, adsorbate_o], num_configurations=num_sites
)
print(f" Generated {len(multi_ads_config_c_o.atoms_list)} configurations")
c_o_energies = []
c_o_energies_ml = []
c_o_energies_d3 = []
c_o_configs = []
for idx, config in enumerate(multi_ads_config_c_o.atoms_list):
config_relaxed = config.copy()
config_relaxed.set_pbc([True, True, True])
config_relaxed.calc = base_calc
opt = LBFGS(config_relaxed, logfile=None)
opt.run(fmax=0.05, steps=relaxation_steps)
# Check C-O bond distance to ensure they haven't formed CO molecule
c_o_dist = config_relaxed[config_relaxed.get_tags() == 2].get_distance(
0, 1, mic=True
)
# CO bond length is ~1.15 Å, so if distance < 1.5 Å, they've formed a molecule
if c_o_dist < 1.5:
print(
f" Config {idx+1}: ⚠ REJECTED - C and O formed CO molecule (d = {c_o_dist:.2f} Å)"
)
continue
E_ml = config_relaxed.get_potential_energy()
config_relaxed.calc = d3_calc
E_d3 = config_relaxed.get_potential_energy()
E_total = E_ml + E_d3
c_o_energies.append(E_total)
c_o_energies_ml.append(E_ml)
c_o_energies_d3.append(E_d3)
c_o_configs.append(config_relaxed)
print(
f" Config {idx+1}: E_total = {E_total:.2f} eV (RPBE: {E_ml:.2f}, D3: {E_d3:.2f}, C-O dist: {c_o_dist:.2f} Å)"
)
best_c_o_idx = np.argmin(c_o_energies)
initial_c_o = c_o_configs[best_c_o_idx]
E_initial_c_o = c_o_energies[best_c_o_idx]
E_initial_c_o_ml = c_o_energies_ml[best_c_o_idx]
E_initial_c_o_d3 = c_o_energies_d3[best_c_o_idx]
print(f"\n → Best C*+O* (Config {best_c_o_idx+1}):")
print(f" RPBE: {E_initial_c_o_ml:.2f} eV")
print(f" D3: {E_initial_c_o_d3:.2f} eV")
print(f" Total: {E_initial_c_o:.2f} eV")
# Save best C+O state
ase.io.write(str(output_dir / part_dirs["part6"] / "co_initial_best.traj"), initial_c_o)
print(" ✓ Best C*+O* structure saved")
# Visualize best C*+O* structure
print("\n Visualizing best C*+O* structure...")
view(initial_c_o, viewer='x3d')
2. Initial State: C* + O* on Ni(111)
Generating C+O configurations...
Generated 5 configurations
Config 1: E_total = -502.79 eV (RPBE: -465.89, D3: -36.90, C-O dist: 4.33 Å)
Config 2: E_total = -502.49 eV (RPBE: -465.60, D3: -36.89, C-O dist: 2.95 Å)
Config 3: E_total = -502.83 eV (RPBE: -465.94, D3: -36.89, C-O dist: 4.33 Å)
Config 4: E_total = -502.49 eV (RPBE: -465.60, D3: -36.89, C-O dist: 2.95 Å)
Config 5: E_total = -502.83 eV (RPBE: -465.94, D3: -36.89, C-O dist: 4.31 Å)
→ Best C*+O* (Config 3):
RPBE: -465.94 eV
D3: -36.89 eV
Total: -502.83 eV
✓ Best C*+O* structure saved
Visualizing best C*+O* structure...
Step 3b: Calculate C* and O* Energies Separately¶
Another strategy to calculate the initial energies for *C and *O at very low coverage (without interactions between the two reactants) is to do two separate relaxations.
# Clean slab
ni_bulk_obj = Bulk(bulk_atoms=ni_bulk_atoms)
clean_slab = Slab.from_bulk_get_specific_millers(
bulk=ni_bulk_obj_c_o, specific_millers=(1, 1, 1)
)[0].atoms
clean_slab.set_pbc([True, True, True])
clean_slab.calc = base_calc
opt = LBFGS(clean_slab, logfile=None)
opt.run(fmax=0.05, steps=relaxation_steps)
E_clean_ml = clean_slab.get_potential_energy()
clean_slab.calc = d3_calc
E_clean_d3 = clean_slab.get_potential_energy()
E_clean = E_clean_ml + E_clean_d3
print(
f"\n Clean slab: E_total = {E_clean:.2f} eV (RPBE: {E_clean_ml:.2f}, D3: {E_clean_d3:.2f})"
)
Clean slab: E_total = -487.46 eV (RPBE: -450.89, D3: -36.57)
print(f"\n2b. Separate C* and O* Energies:")
print(" Calculating energies in separate unit cells to avoid interactions")
ni_bulk_obj_c_o = Bulk(bulk_atoms=ni_bulk_atoms)
ni_slab_c_o = Slab.from_bulk_get_specific_millers(
bulk=ni_bulk_obj_c_o, specific_millers=(1, 1, 1)
)[0]
print("\n Generating C* configurations...")
multi_ads_config_c = MultipleAdsorbateSlabConfig(
ni_slab_c_o,
adsorbates=[Adsorbate(adsorbate_smiles_from_db="*C")],
num_configurations=num_sites,
)
c_energies = []
c_energies_ml = []
c_energies_d3 = []
c_configs = []
for idx, config in enumerate(multi_ads_config_c.atoms_list):
config_relaxed = config.copy()
config_relaxed.set_pbc([True, True, True])
config_relaxed.calc = base_calc
opt = LBFGS(config_relaxed, logfile=None)
opt.run(fmax=0.05, steps=relaxation_steps)
E_ml = config_relaxed.get_potential_energy()
config_relaxed.calc = d3_calc
E_d3 = config_relaxed.get_potential_energy()
E_total = E_ml + E_d3
c_energies.append(E_total)
c_energies_ml.append(E_ml)
c_energies_d3.append(E_d3)
c_configs.append(config_relaxed)
print(
f" Config {idx+1}: E_total = {E_total:.2f} eV (RPBE: {E_ml:.2f}, D3: {E_d3:.2f})"
)
best_c_idx = np.argmin(c_energies)
c_ads = c_configs[best_c_idx]
E_c = c_energies[best_c_idx]
E_c_ml = c_energies_ml[best_c_idx]
E_c_d3 = c_energies_d3[best_c_idx]
print(f"\n → Best C* (Config {best_c_idx+1}):")
print(f" RPBE: {E_c_ml:.2f} eV")
print(f" D3: {E_c_d3:.2f} eV")
print(f" Total: {E_c:.2f} eV")
# Save best C state
ase.io.write(str(output_dir / part_dirs["part6"] / "c_best.traj"), c_ads)
# Visualize best C* structure
print("\n Visualizing best C* structure...")
view(c_ads, viewer='x3d')
# Generate O* configuration
print("\n Generating O* configurations...")
multi_ads_config_o = MultipleAdsorbateSlabConfig(
ni_slab_c_o,
adsorbates=[Adsorbate(adsorbate_smiles_from_db="*O")],
num_configurations=num_sites,
)
o_energies = []
o_energies_ml = []
o_energies_d3 = []
o_configs = []
for idx, config in enumerate(multi_ads_config_o.atoms_list):
config_relaxed = config.copy()
config_relaxed.set_pbc([True, True, True])
config_relaxed.calc = base_calc
opt = LBFGS(config_relaxed, logfile=None)
opt.run(fmax=0.05, steps=relaxation_steps)
E_ml = config_relaxed.get_potential_energy()
config_relaxed.calc = d3_calc
E_d3 = config_relaxed.get_potential_energy()
E_total = E_ml + E_d3
o_energies.append(E_total)
o_energies_ml.append(E_ml)
o_energies_d3.append(E_d3)
o_configs.append(config_relaxed)
print(
f" Config {idx+1}: E_total = {E_total:.2f} eV (RPBE: {E_ml:.2f}, D3: {E_d3:.2f})"
)
best_o_idx = np.argmin(o_energies)
o_ads = o_configs[best_o_idx]
E_o = o_energies[best_o_idx]
E_o_ml = o_energies_ml[best_o_idx]
E_o_d3 = o_energies_d3[best_o_idx]
print(f"\n → Best O* (Config {best_o_idx+1}):")
print(f" RPBE: {E_o_ml:.2f} eV")
print(f" D3: {E_o_d3:.2f} eV")
print(f" Total: {E_o:.2f} eV")
# Save best O state
ase.io.write(str(output_dir / part_dirs["part6"] / "o_best.traj"), o_ads)
# Visualize best O* structure
print("\n Visualizing best O* structure...")
view(o_ads, viewer='x3d')
# Calculate combined energy for separate C* and O*
E_initial_c_o_separate = E_c + E_o
E_initial_c_o_separate_ml = E_c_ml + E_o_ml
E_initial_c_o_separate_d3 = E_c_d3 + E_o_d3
2b. Separate C* and O* Energies:
Calculating energies in separate unit cells to avoid interactions
Generating C* configurations...
Config 1: E_total = -495.73 eV (RPBE: -458.94, D3: -36.79)
Config 2: E_total = -495.73 eV (RPBE: -458.94, D3: -36.79)
Config 3: E_total = -495.73 eV (RPBE: -458.94, D3: -36.78)
Config 4: E_total = -495.67 eV (RPBE: -458.89, D3: -36.78)
Config 5: E_total = -495.73 eV (RPBE: -458.94, D3: -36.79)
→ Best C* (Config 5):
RPBE: -458.94 eV
D3: -36.79 eV
Total: -495.73 eV
Visualizing best C* structure...
Generating O* configurations...
Config 1: E_total = -494.56 eV (RPBE: -457.87, D3: -36.68)
Config 2: E_total = -494.55 eV (RPBE: -457.88, D3: -36.68)
Config 3: E_total = -494.55 eV (RPBE: -457.87, D3: -36.68)
Config 4: E_total = -494.66 eV (RPBE: -457.97, D3: -36.69)
Config 5: E_total = -494.55 eV (RPBE: -457.88, D3: -36.68)
→ Best O* (Config 4):
RPBE: -457.97 eV
D3: -36.69 eV
Total: -494.66 eV
Visualizing best O* structure...
print(f"\n Combined C* + O* (separate calculations):")
print(f" RPBE: {E_initial_c_o_separate_ml:.2f} eV")
print(f" D3: {E_initial_c_o_separate_d3:.2f} eV")
print(f" Total: {E_initial_c_o_separate:.2f} eV")
print(f"\n Comparison:")
print(f" C*+O* (same cell): {E_initial_c_o - E_clean:.2f} eV")
print(f" C* + O* (separate): {E_initial_c_o_separate - 2*E_clean:.2f} eV")
print(
f" Difference: {(E_initial_c_o - E_clean) - (E_initial_c_o_separate - 2*E_clean):.2f} eV"
)
print(" ✓ Separate C* and O* energies calculated")
Combined C* + O* (separate calculations):
RPBE: -916.91 eV
D3: -73.47 eV
Total: -990.39 eV
Comparison:
C*+O* (same cell): -15.37 eV
C* + O* (separate): -15.47 eV
Difference: 0.10 eV
✓ Separate C* and O* energies calculated
Step 4: Calculate Reaction Energy with ZPE¶
Compute the thermochemistry for C* + O* → CO* with ZPE corrections:
print(f"\n3. Reaction Energy (C* + O* → CO*):")
print(f" " + "=" * 60)
# Electronic energies
print(f"\n Electronic Energies:")
print(
f" Initial (C*+O*): RPBE = {E_initial_c_o_ml:.2f} eV, D3 = {E_initial_c_o_d3:.2f} eV, Total = {E_initial_c_o:.2f} eV"
)
print(
f" Final (CO*): RPBE = {E_final_co_ml:.2f} eV, D3 = {E_final_co_d3:.2f} eV, Total = {E_final_co:.2f} eV"
)
# Reaction energies without ZPE
delta_E_rpbe = E_final_co_ml - E_initial_c_o_ml
delta_E_d3_contrib = E_final_co_d3 - E_initial_c_o_d3
delta_E_elec = E_final_co - E_initial_c_o
print(f"\n Reaction Energies (without ZPE):")
print(f" ΔE(RPBE only): {delta_E_rpbe:.2f} eV = {delta_E_rpbe*96.485:.1f} kJ/mol")
print(
f" ΔE(D3 contrib): {delta_E_d3_contrib:.2f} eV = {delta_E_d3_contrib*96.485:.1f} kJ/mol"
)
print(f" ΔE(RPBE+D3): {delta_E_elec:.2f} eV = {delta_E_elec*96.485:.1f} kJ/mol")
# Calculate ZPE for CO* (final state)
print(f"\n Computing ZPE for CO*...")
final_co.calc = base_calc
co_indices = np.where(final_co.get_tags() == 2)[0]
vib_co = Vibrations(final_co, indices=co_indices, delta=0.02, name="vib_co")
vib_co.run()
vib_energies_co = vib_co.get_energies()
zpe_co = np.sum(vib_energies_co[vib_energies_co > 0]) / 2.0
vib_co.clean()
print(f" ZPE(CO*): {zpe_co:.2f} eV ({zpe_co*1000:.1f} meV)")
# Calculate ZPE for C* and O* (initial state)
print(f"\n Computing ZPE for C* and O*...")
initial_c_o.calc = base_calc
c_o_indices = np.where(initial_c_o.get_tags() == 2)[0]
vib_c_o = Vibrations(initial_c_o, indices=c_o_indices, delta=0.02, name="vib_c_o")
vib_c_o.run()
vib_energies_c_o = vib_c_o.get_energies()
zpe_c_o = np.sum(vib_energies_c_o[vib_energies_c_o > 0]) / 2.0
vib_c_o.clean()
print(f" ZPE(C*+O*): {zpe_c_o:.2f} eV ({zpe_c_o*1000:.1f} meV)")
# Total reaction energy with ZPE
delta_zpe = zpe_co - zpe_c_o
delta_E_zpe = delta_E_elec + delta_zpe
print(f"\n Reaction Energy (with ZPE):")
print(f" ΔE(electronic): {delta_E_elec:.2f} eV = {delta_E_elec*96.485:.1f} kJ/mol")
print(
f" ΔZPE: {delta_zpe:.2f} eV = {delta_zpe*96.485:.1f} kJ/mol ({delta_zpe*1000:.1f} meV)"
)
print(f" ΔE(total): {delta_E_zpe:.2f} eV = {delta_E_zpe*96.485:.1f} kJ/mol")
print(f"\n Summary:")
print(
f" Without D3, without ZPE: {delta_E_rpbe:.2f} eV = {delta_E_rpbe*96.485:.1f} kJ/mol"
)
print(
f" With D3, without ZPE: {delta_E_elec:.2f} eV = {delta_E_elec*96.485:.1f} kJ/mol"
)
print(
f" With D3, with ZPE: {delta_E_zpe:.2f} eV = {delta_E_zpe*96.485:.1f} kJ/mol"
)
print(f"\n " + "=" * 60)
print(f"\n Comparison with Paper (Table 5):")
print(f" Paper (DFT-D3): -142.7 kJ/mol = -1.48 eV")
print(f" This work: {delta_E_zpe*96.485:.1f} kJ/mol = {delta_E_zpe:.2f} eV")
print(f" Difference: {abs(delta_E_zpe - (-1.48)):.2f} eV")
if delta_E_zpe < 0:
print(f"\n ✓ Reaction is exothermic (C+O recombination favorable)")
else:
print(f"\n ⚠ Reaction is endothermic (dissociation favorable)")
3. Reaction Energy (C* + O* → CO*):
============================================================
Electronic Energies:
Initial (C*+O*): RPBE = -465.94 eV, D3 = -36.89 eV, Total = -502.83 eV
Final (CO*): RPBE = -467.14 eV, D3 = -36.82 eV, Total = -503.97 eV
Reaction Energies (without ZPE):
ΔE(RPBE only): -1.21 eV = -116.5 kJ/mol
ΔE(D3 contrib): 0.07 eV = 6.7 kJ/mol
ΔE(RPBE+D3): -1.14 eV = -109.8 kJ/mol
Computing ZPE for CO*...
ZPE(CO*): 0.18+0.00j eV (182.4+0.0j meV)
Computing ZPE for C* and O*...
ZPE(C*+O*): 0.17+0.00j eV (174.0+0.0j meV)
Reaction Energy (with ZPE):
ΔE(electronic): -1.14 eV = -109.8 kJ/mol
ΔZPE: 0.01+0.00j eV = 0.8+0.0j kJ/mol (8.3+0.0j meV)
ΔE(total): -1.13+0.00j eV = -109.0+0.0j kJ/mol
Summary:
Without D3, without ZPE: -1.21 eV = -116.5 kJ/mol
With D3, without ZPE: -1.14 eV = -109.8 kJ/mol
With D3, with ZPE: -1.13+0.00j eV = -109.0+0.0j kJ/mol
============================================================
Comparison with Paper (Table 5):
Paper (DFT-D3): -142.7 kJ/mol = -1.48 eV
This work: -109.0+0.0j kJ/mol = -1.13+0.00j eV
Difference: 0.35 eV
✓ Reaction is exothermic (C+O recombination favorable)
Step 5: Calculate CO Adsorption Energy (Bonus)¶
Calculate how strongly CO binds to the surface:
print(f"\n4. CO Adsorption Energy ( CO(g) + * → CO*):")
print(" This helps us understand CO binding strength")
# CO(g)
co_gas = Atoms("CO", positions=[[0, 0, 0], [0, 0, 1.15]])
co_gas.center(vacuum=10.0)
co_gas.set_pbc([True, True, True])
co_gas.calc = base_calc
opt = LBFGS(co_gas, logfile=None)
opt.run(fmax=0.05, steps=relaxation_steps)
E_co_gas_ml = co_gas.get_potential_energy()
co_gas.calc = d3_calc
E_co_gas_d3 = co_gas.get_potential_energy()
E_co_gas = E_co_gas_ml + E_co_gas_d3
print(
f" CO(g): E_total = {E_co_gas:.2f} eV (RPBE: {E_co_gas_ml:.2f}, D3: {E_co_gas_d3:.2f})"
)
# Calculate ZPE for CO(g)
co_gas.calc = base_calc
vib_co_gas = Vibrations(co_gas, indices=[0, 1], delta=0.01, nfree=2)
vib_co_gas.clean()
vib_co_gas.run()
vib_energies_co_gas = vib_co_gas.get_energies()
zpe_co_gas = 0.5 * np.sum(vib_energies_co_gas[vib_energies_co_gas > 0])
vib_co_gas.clean()
print(f" ZPE(CO(g)): {zpe_co_gas:.2f} eV")
print(f" ZPE(CO*): {zpe_co:.2f} eV (from Step 4 calculation)")
# Electronic adsorption energy
E_ads_co_elec = E_final_co - E_clean - E_co_gas
# ZPE contribution to adsorption energy
delta_zpe_ads = zpe_co - zpe_co_gas
# Total adsorption energy with ZPE
E_ads_co_total = E_ads_co_elec + delta_zpe_ads
print(f"\n Electronic Energy Breakdown:")
print(f" ΔE(RPBE only) = {(E_final_co_ml - E_clean_ml - E_co_gas_ml):.2f} eV")
print(f" ΔE(D3 contrib) = {((E_final_co_d3 - E_clean_d3 - E_co_gas_d3)):.2f} eV")
print(f" ΔE(RPBE+D3) = {E_ads_co_elec:.2f} eV")
print(f"\n ZPE Contribution:")
print(f" ΔZPE = {delta_zpe_ads:.2f} eV")
print(f"\n Total Adsorption Energy:")
print(f" ΔE(total) = {E_ads_co_total:.2f} eV = {E_ads_co_total*96.485:.1f} kJ/mol")
print(f"\n Summary:")
print(
f" E_ads(CO) without ZPE = {-E_ads_co_elec:.2f} eV = {-E_ads_co_elec*96.485:.1f} kJ/mol"
)
print(
f" E_ads(CO) with ZPE = {-E_ads_co_total:.2f} eV = {-E_ads_co_total*96.485:.1f} kJ/mol"
)
print(
f" → CO binds {abs(E_ads_co_total):.2f} eV stronger than H ({abs(E_ads_co_total)/0.60:.1f}x)"
)
4. CO Adsorption Energy ( CO(g) + * → CO*):
This helps us understand CO binding strength
CO(g): E_total = -14.43 eV (RPBE: -14.42, D3: -0.01)
ZPE(CO(g)): 0.13+0.00j eV
ZPE(CO*): 0.18+0.00j eV (from Step 4 calculation)
Electronic Energy Breakdown:
ΔE(RPBE only) = -1.84 eV
ΔE(D3 contrib) = -0.24 eV
ΔE(RPBE+D3) = -2.08 eV
ZPE Contribution:
ΔZPE = 0.05-0.00j eV
Total Adsorption Energy:
ΔE(total) = -2.03-0.00j eV = -195.7-0.0j kJ/mol
Summary:
E_ads(CO) without ZPE = 2.08 eV = 200.6 kJ/mol
E_ads(CO) with ZPE = 2.03+0.00j eV = 195.7+0.0j kJ/mol
→ CO binds 2.03 eV stronger than H (3.4x)
Step 6: Find guesses for nearby initial and final states for the reaction¶
Now that we have an estimate on the reaction energy from the best possible initial and final states, we want to find a transition state (barrier) for this reaction. There are MANY possible ways that we could do this. In this case, we’ll start with the *CO final state and then try and find a nearby local minimal of *C and *O, by fixing the C-O bond distance and finding a nearby local minima. Note that this approach required some insight into what the transition state might look like, and could be considerably more complicated for a reaction that did not involve breaking a single bond.
print(f"\nFinding Transition State Initial and Final States")
print(" Creating initial guess with stretched C-O bond...")
print(" Starting from CO* and stretching the C-O bond...")
# Create a guess structure with stretched CO bond (start from CO*)
initial_guess = final_co.copy()
# Set up a constraint to fix the bond length to ~2 Angstroms, which should be far enough that we'll be closer to *C+*O than *CO
co_indices = np.where(initial_guess.get_tags() == 2)[0]
# Rotate the atoms a bit just to break the symmetry and prevent the O from going straight up to satisfy the constraint
initial_slab = initial_guess[initial_guess.get_tags() != 2]
initial_co = initial_guess[initial_guess.get_tags() == 2]
initial_co.rotate(30, "x", center=initial_co.positions[0])
initial_guess = initial_slab + initial_co
initial_guess.calc = FAIRChemCalculator(predictor, task_name="oc20")
# Add constraints to keep the CO bond length extended
initial_guess.constraints += [
FixBondLengths([co_indices], tolerance=1e-2, iterations=5000, bondlengths=[2.0])
]
try:
opt = LBFGS(
initial_guess,
trajectory=output_dir / part_dirs["part6"] / "initial_guess_with_constraint.traj",
)
opt.run(fmax=0.01)
except RuntimeError:
# The FixBondLength constraint is sometimes a little finicky,
# but it's ok if it doesn't finish as it's just an initial guess
# for the next step
pass
# Now that we have a guess, re-relax without the constraints
initial_guess.constraints = initial_guess.constraints[:-1]
opt = LBFGS(
initial_guess,
trajectory=output_dir
/ part_dirs["part6"]
/ "initial_guess_without_constraint.traj",
)
opt.run(fmax=0.01)
Finding Transition State Initial and Final States
Creating initial guess with stretched C-O bond...
Starting from CO* and stretching the C-O bond...
Step Time Energy fmax
LBFGS: 0 22:58:02 -466.834893 0.917781
LBFGS: 1 22:58:02 -461.448925 4.314657
LBFGS: 2 22:58:02 -461.516199 3.926632
LBFGS: 3 22:58:03 -461.855306 0.986093
LBFGS: 4 22:58:03 -461.926683 0.964224
LBFGS: 5 22:58:04 -461.951793 0.968209
LBFGS: 6 22:58:04 -461.943270 0.797500
LBFGS: 7 22:58:05 -461.999506 0.461175
LBFGS: 8 22:58:05 -461.949957 0.387598
LBFGS: 9 22:58:06 -462.013023 0.989769
LBFGS: 10 22:58:06 -462.020538 0.416511
LBFGS: 11 22:58:06 -462.035904 0.507579
LBFGS: 12 22:58:07 -461.983784 0.681752
LBFGS: 13 22:58:07 -462.119673 1.549978
LBFGS: 14 22:58:08 -462.248276 3.000015
LBFGS: 15 22:58:08 -462.271553 4.133749
LBFGS: 16 22:58:09 -462.265650 4.494072
LBFGS: 17 22:58:09 -462.284794 4.429908
LBFGS: 18 22:58:10 -462.447279 3.275189
LBFGS: 19 22:58:10 -462.519708 2.699714
LBFGS: 20 22:58:10 -462.597811 2.099330
LBFGS: 21 22:58:11 -462.747985 1.033713
LBFGS: 22 22:58:11 -462.782783 0.970312
LBFGS: 23 22:58:12 -462.819867 0.673961
LBFGS: 24 22:58:12 -462.860431 0.552251
LBFGS: 25 22:58:12 -462.884325 0.473527
LBFGS: 26 22:58:13 -462.904703 0.345437
LBFGS: 27 22:58:13 -462.932099 0.178797
LBFGS: 28 22:58:13 -462.929345 0.304588
LBFGS: 29 22:58:14 -462.954871 0.344229
LBFGS: 30 22:58:14 -462.949750 0.736852
LBFGS: 31 22:58:15 -462.957402 0.513627
LBFGS: 32 22:58:15 -462.970321 0.355836
LBFGS: 33 22:58:15 -462.977351 0.274754
LBFGS: 34 22:58:16 -463.010629 0.250257
LBFGS: 35 22:58:16 -463.002233 0.283726
LBFGS: 36 22:58:17 -463.009702 0.370231
LBFGS: 37 22:58:17 -463.022824 0.357284
LBFGS: 38 22:58:17 -463.056231 0.475199
LBFGS: 39 22:58:18 -463.080709 0.364074
LBFGS: 40 22:58:18 -463.100479 0.332826
LBFGS: 41 22:58:19 -463.117813 0.366778
LBFGS: 42 22:58:19 -463.147498 0.390241
LBFGS: 43 22:58:20 -463.157726 0.468219
LBFGS: 44 22:58:20 -463.180632 0.453781
LBFGS: 45 22:58:20 -463.192985 0.469546
LBFGS: 46 22:58:21 -463.213815 0.458909
LBFGS: 47 22:58:21 -463.241082 0.437740
LBFGS: 48 22:58:21 -463.252927 0.350417
LBFGS: 49 22:58:22 -463.277174 0.283775
LBFGS: 50 22:58:22 -463.288961 0.284282
LBFGS: 51 22:58:23 -463.303655 0.312533
LBFGS: 52 22:58:23 -463.315095 0.417053
LBFGS: 53 22:58:24 -463.329794 0.557971
LBFGS: 54 22:58:24 -463.357241 0.915807
LBFGS: 55 22:58:24 -463.322921 1.140771
LBFGS: 56 22:58:25 -463.318368 1.126206
LBFGS: 57 22:58:25 -463.226094 2.128311
LBFGS: 58 22:58:26 -463.262780 1.867984
LBFGS: 59 22:58:26 -463.294811 1.626560
LBFGS: 60 22:58:26 -463.322780 1.385563
LBFGS: 61 22:58:27 -463.344361 1.163193
LBFGS: 62 22:58:27 -463.361243 0.960958
LBFGS: 63 22:58:28 -463.383046 0.792259
LBFGS: 64 22:58:28 -463.397775 0.831910
LBFGS: 65 22:58:29 -463.447647 0.795115
LBFGS: 66 22:58:29 -463.498051 0.869946
LBFGS: 67 22:58:29 -463.522879 0.735191
LBFGS: 68 22:58:30 -463.558352 0.662734
LBFGS: 69 22:58:30 -463.699651 0.657746
LBFGS: 70 22:58:31 -463.722788 0.537573
LBFGS: 71 22:58:31 -463.749039 0.445496
LBFGS: 72 22:58:32 -463.761725 0.500191
LBFGS: 73 22:58:32 -463.781449 0.443218
LBFGS: 74 22:58:33 -463.821717 0.680057
LBFGS: 75 22:58:33 -463.843169 0.618735
LBFGS: 76 22:58:33 -463.864649 0.669504
LBFGS: 77 22:58:34 -463.900301 0.817710
LBFGS: 78 22:58:34 -463.925509 0.875651
LBFGS: 79 22:58:35 -463.966101 0.723712
LBFGS: 80 22:58:35 -463.993787 0.818436
LBFGS: 81 22:58:36 -463.975498 1.002289
LBFGS: 82 22:58:36 -463.987427 1.040322
LBFGS: 83 22:58:37 -463.981015 1.096524
LBFGS: 84 22:58:37 -463.987178 1.124840
LBFGS: 85 22:58:37 -464.010992 0.567055
LBFGS: 86 22:58:38 -464.024430 0.496483
LBFGS: 87 22:58:38 -464.024839 1.042720
LBFGS: 88 22:58:39 -464.039686 0.781510
LBFGS: 89 22:58:39 -464.006519 0.950455
LBFGS: 90 22:58:39 -464.027956 0.809876
LBFGS: 91 22:58:40 -464.049066 0.611221
LBFGS: 92 22:58:40 -464.086840 0.383218
LBFGS: 93 22:58:41 -464.096469 0.300330
LBFGS: 94 22:58:41 -464.117299 0.203387
LBFGS: 95 22:58:41 -464.131803 0.209977
LBFGS: 96 22:58:42 -464.139375 0.173414
LBFGS: 97 22:58:42 -464.139471 0.157549
LBFGS: 98 22:58:43 -464.144575 0.133294
LBFGS: 99 22:58:43 -464.144899 0.138822
LBFGS: 100 22:58:43 -464.152330 0.195996
LBFGS: 101 22:58:44 -464.149471 0.154909
LBFGS: 102 22:58:44 -464.148448 0.119949
LBFGS: 103 22:58:44 -464.157938 0.126427
LBFGS: 104 22:58:45 -464.160187 0.147873
LBFGS: 105 22:58:45 -464.156060 0.158662
LBFGS: 106 22:58:45 -464.146233 0.241200
LBFGS: 107 22:58:46 -464.109099 0.493838
LBFGS: 108 22:58:46 -464.148840 0.164830
LBFGS: 109 22:58:47 -464.159488 0.081885
LBFGS: 110 22:58:47 -464.160220 0.042476
LBFGS: 111 22:58:47 -464.155117 0.070289
LBFGS: 112 22:58:48 -464.156110 0.251864
LBFGS: 113 22:58:48 -464.160669 0.045135
LBFGS: 114 22:58:48 -464.161932 0.045899
LBFGS: 115 22:58:49 -464.155172 0.112271
LBFGS: 116 22:58:49 -464.165756 0.074095
LBFGS: 117 22:58:49 -464.162830 0.017740
LBFGS: 118 22:58:50 -464.154241 0.095318
LBFGS: 119 22:58:50 -464.158255 0.041547
LBFGS: 120 22:58:51 -464.157915 0.042219
LBFGS: 121 22:58:51 -464.162330 0.046682
LBFGS: 122 22:58:51 -464.163276 0.044583
LBFGS: 123 22:58:52 -464.162115 0.015688
LBFGS: 124 22:58:52 -464.161470 0.005963
Step Time Energy fmax
LBFGS: 0 22:58:52 -464.161470 0.474727
LBFGS: 1 22:58:52 -464.167537 0.445871
LBFGS: 2 22:58:53 -464.194979 0.861553
LBFGS: 3 22:58:53 -464.207196 0.514678
LBFGS: 4 22:58:53 -464.233530 0.344611
LBFGS: 5 22:58:54 -464.253483 0.429083
LBFGS: 6 22:58:54 -464.260649 0.333534
LBFGS: 7 22:58:54 -464.273046 0.267175
LBFGS: 8 22:58:55 -464.277242 0.243616
LBFGS: 9 22:58:55 -464.285951 0.187889
LBFGS: 10 22:58:56 -464.287925 0.099265
LBFGS: 11 22:58:56 -464.288962 0.085529
LBFGS: 12 22:58:56 -464.289782 0.098488
LBFGS: 13 22:58:57 -464.290858 0.109136
LBFGS: 14 22:58:57 -464.291599 0.074870
LBFGS: 15 22:58:57 -464.292005 0.052161
LBFGS: 16 22:58:58 -464.292279 0.064658
LBFGS: 17 22:58:58 -464.292619 0.074097
LBFGS: 18 22:58:59 -464.293029 0.062528
LBFGS: 19 22:58:59 -464.293367 0.052944
LBFGS: 20 22:58:59 -464.293534 0.027976
LBFGS: 21 22:59:00 -464.293627 0.023988
LBFGS: 22 22:59:00 -464.293711 0.035538
LBFGS: 23 22:59:01 -464.293810 0.037807
LBFGS: 24 22:59:01 -464.293890 0.025850
LBFGS: 25 22:59:01 -464.293945 0.019578
LBFGS: 26 22:59:02 -464.293988 0.021480
LBFGS: 27 22:59:02 -464.294047 0.031322
LBFGS: 28 22:59:02 -464.294114 0.030995
LBFGS: 29 22:59:03 -464.294159 0.025794
LBFGS: 30 22:59:03 -464.294192 0.025214
LBFGS: 31 22:59:03 -464.294236 0.023524
LBFGS: 32 22:59:04 -464.294291 0.038134
LBFGS: 33 22:59:04 -464.294400 0.051076
LBFGS: 34 22:59:05 -464.294566 0.051741
LBFGS: 35 22:59:05 -464.294816 0.052633
LBFGS: 36 22:59:05 -464.295251 0.074403
LBFGS: 37 22:59:06 -464.296864 0.252892
LBFGS: 38 22:59:06 -464.295733 0.953157
LBFGS: 39 22:59:06 -464.298127 0.423118
LBFGS: 40 22:59:07 -464.298866 0.544225
LBFGS: 41 22:59:07 -464.296773 1.338418
LBFGS: 42 22:59:08 -464.300521 0.733056
LBFGS: 43 22:59:08 -464.301723 0.831436
LBFGS: 44 22:59:08 -464.304847 1.621276
LBFGS: 45 22:59:09 -464.306881 1.235903
LBFGS: 46 22:59:09 -464.308666 1.303208
LBFGS: 47 22:59:09 -464.316115 1.654947
LBFGS: 48 22:59:10 -464.318170 1.526813
LBFGS: 49 22:59:10 -464.320655 1.547132
LBFGS: 50 22:59:10 -464.336473 1.713104
LBFGS: 51 22:59:11 -464.345251 1.589201
LBFGS: 52 22:59:11 -464.360104 1.573368
LBFGS: 53 22:59:11 -464.387285 1.392333
LBFGS: 54 22:59:12 -464.461287 1.550169
LBFGS: 55 22:59:12 -464.508443 1.679898
LBFGS: 56 22:59:12 -464.650497 1.735133
LBFGS: 57 22:59:13 -464.968522 1.629692
LBFGS: 58 22:59:13 -465.133245 1.183350
LBFGS: 59 22:59:14 -464.450435 4.352140
LBFGS: 60 22:59:14 -465.106725 1.445714
LBFGS: 61 22:59:14 -465.220301 1.567471
LBFGS: 62 22:59:15 -465.374637 0.774904
LBFGS: 63 22:59:15 -465.397749 0.709250
LBFGS: 64 22:59:15 -465.437779 0.578970
LBFGS: 65 22:59:16 -465.486601 0.481269
LBFGS: 66 22:59:16 -465.525107 0.367138
LBFGS: 67 22:59:17 -465.552917 0.611140
LBFGS: 68 22:59:17 -465.577937 0.763966
LBFGS: 69 22:59:17 -465.623060 0.948063
LBFGS: 70 22:59:18 -465.654885 0.960019
LBFGS: 71 22:59:18 -465.685228 0.957109
LBFGS: 72 22:59:18 -465.699798 0.927262
LBFGS: 73 22:59:19 -465.712257 0.971257
LBFGS: 74 22:59:19 -465.721645 0.827033
LBFGS: 75 22:59:19 -465.730674 0.836305
LBFGS: 76 22:59:20 -465.737317 0.787170
LBFGS: 77 22:59:20 -465.741986 0.768955
LBFGS: 78 22:59:21 -465.748592 0.760080
LBFGS: 79 22:59:21 -465.753596 0.760215
LBFGS: 80 22:59:22 -465.758899 0.756206
LBFGS: 81 22:59:22 -465.765989 0.742395
LBFGS: 82 22:59:22 -465.775758 0.698412
LBFGS: 83 22:59:23 -465.786572 0.610960
LBFGS: 84 22:59:23 -465.794832 0.492479
LBFGS: 85 22:59:23 -465.800587 0.421308
LBFGS: 86 22:59:24 -465.806902 0.380507
LBFGS: 87 22:59:24 -465.814588 0.350476
LBFGS: 88 22:59:24 -465.820748 0.297331
LBFGS: 89 22:59:25 -465.824080 0.244503
LBFGS: 90 22:59:25 -465.826316 0.207310
LBFGS: 91 22:59:25 -465.828427 0.176439
LBFGS: 92 22:59:26 -465.830748 0.137518
LBFGS: 93 22:59:26 -465.832745 0.120022
LBFGS: 94 22:59:26 -465.833824 0.077618
LBFGS: 95 22:59:27 -465.834363 0.082230
LBFGS: 96 22:59:27 -465.834800 0.093101
LBFGS: 97 22:59:27 -465.835309 0.098392
LBFGS: 98 22:59:28 -465.835896 0.090032
LBFGS: 99 22:59:28 -465.836467 0.068120
LBFGS: 100 22:59:29 -465.836934 0.056416
LBFGS: 101 22:59:29 -465.837304 0.034917
LBFGS: 102 22:59:29 -465.837632 0.038862
LBFGS: 103 22:59:30 -465.837959 0.039841
LBFGS: 104 22:59:30 -465.838259 0.038954
LBFGS: 105 22:59:31 -465.838514 0.036005
LBFGS: 106 22:59:31 -465.838694 0.025839
LBFGS: 107 22:59:31 -465.838804 0.017330
LBFGS: 108 22:59:32 -465.838868 0.014943
LBFGS: 109 22:59:32 -465.838910 0.012543
LBFGS: 110 22:59:32 -465.838943 0.010813
LBFGS: 111 22:59:33 -465.838970 0.009830
np.True_Step 7: Run NEB to Find Activation Barrier¶
Use the nudged elastic band method to find the minimum energy path:
print(f"\n7. NEB Barrier Calculation (C* + O* → CO*)")
print(" Setting up 7-image NEB chain with TS guess in middle...")
print(" Reaction: C* + O* (initial) → TS → CO* (final)")
initial = initial_guess.copy()
initial.calc = FAIRChemCalculator(predictor, task_name="oc20")
images = [initial] # Start with C* + O*
n_images = 10
for i in range(n_images):
image = initial.copy()
image.calc = FAIRChemCalculator(predictor, task_name="oc20")
images.append(image)
final = final_co.copy()
final.calc = FAIRChemCalculator(predictor, task_name="oc20")
images.append(final) # End with CO*
# Interpolate with better initial guess
dyneb = DyNEB(images, climb=True, fmax=0.05)
# Interpolate first half (C*+O* → TS)
print("\n Interpolating images...")
dyneb.interpolate("idpp", mic=True)
# Optimize
print(" Optimizing NEB path (this may take a while)...")
opt = FIRE(
dyneb,
trajectory=str(output_dir / part_dirs["part6"] / "neb.traj"),
logfile=str(output_dir / part_dirs["part6"] / "neb.log"),
)
opt.run(fmax=0.1, steps=relaxation_steps)
# Extract barrier (from C*+O* to TS)
energies = [img.get_potential_energy() for img in images]
energies_rel = np.array(energies) - energies[0]
E_barrier = np.max(energies_rel)
print(f"\n ✓ NEB converged!")
print(
f"\n Forward barrier (C*+O* → CO*): {E_barrier:.2f} eV = {E_barrier*96.485:.1f} kJ/mol"
)
print(
f" Reverse barrier (CO* → C*+O*): {E_barrier - energies_rel[-1]:.2f} eV = {(E_barrier- energies_rel[-1])*96.485:.1f} kJ/mol"
)
print(f"\n Paper (Table 5): 153 kJ/mol = 1.59 eV ")
print(f" Difference: {abs(E_barrier - 1.59):.2f} eV")
7. NEB Barrier Calculation (C* + O* → CO*)
Setting up 7-image NEB chain with TS guess in middle...
Reaction: C* + O* (initial) → TS → CO* (final)
Interpolating images...
Optimizing NEB path (this may take a while)...
✓ NEB converged!
Forward barrier (C*+O* → CO*): 1.60 eV = 154.8 kJ/mol
Reverse barrier (CO* → C*+O*): 2.91 eV = 280.6 kJ/mol
Paper (Table 5): 153 kJ/mol = 1.59 eV
Difference: 0.01 eV
Step 8: Visualize NEB Path and Key Structures¶
Create plots showing the reaction pathway:
print("\n Creating NEB visualization...")
# Plot NEB path
fig, ax = plt.subplots(figsize=(10, 6))
ax.plot(
range(len(energies_rel)),
energies_rel,
"o-",
linewidth=2,
markersize=10,
color="steelblue",
label="NEB Path",
)
ax.axhline(0, color="green", linestyle="--", alpha=0.5, label="Initial: C*+O*")
ax.axhline(delta_E_zpe, color="red", linestyle="--", alpha=0.5, label="Final: CO*")
ax.axhline(
E_barrier,
color="orange",
linestyle=":",
alpha=0.7,
linewidth=2,
label=f"Forward Barrier = {E_barrier:.2f} eV",
)
# Annotate transition state
ts_idx = np.argmax(energies_rel)
ax.annotate(
f"TS\n{energies_rel[ts_idx]:.2f} eV",
xy=(ts_idx, energies_rel[ts_idx]),
xytext=(ts_idx, energies_rel[ts_idx] + 0.3),
ha="center",
fontsize=11,
fontweight="bold",
arrowprops=dict(arrowstyle="->", lw=1.5, color="red"),
)
ax.set_xlabel("Image Number", fontsize=13)
ax.set_ylabel("Relative Energy (eV)", fontsize=13)
ax.set_title(
"CO Formation on Ni(111): C* + O* → CO* - NEB Path", fontsize=15, fontweight="bold"
)
ax.legend(fontsize=11, loc="upper left")
ax.grid(True, alpha=0.3)
plt.tight_layout()
plt.savefig(
str(output_dir / part_dirs["part6"] / "neb_path.png"), dpi=300, bbox_inches="tight"
)
plt.show()
# Create animation of NEB path
print("\n Creating NEB path animation...")
from ase.io import write as ase_write
ase.io.write(
str(output_dir / part_dirs["part6"] / "neb_path.gif"), images, format="gif"
)
print(" → Saved as neb_path.gif")
# Visualize key structures
print("\n Visualizing initial state (C* + O*)...")
view(initial_c_o, viewer='x3d')
print("\n Visualizing transition state...")
view(images[ts_idx], viewer='x3d')
print("\n Visualizing final state (CO*)...")
view(final_co, viewer='x3d')
print("\n✓ NEB analysis complete!")
Creating NEB visualization...
/home/runner/work/_tool/Python/3.12.12/x64/lib/python3.12/site-packages/matplotlib/cbook.py:1355: ComplexWarning: Casting complex values to real discards the imaginary part
return np.asarray(x, float)
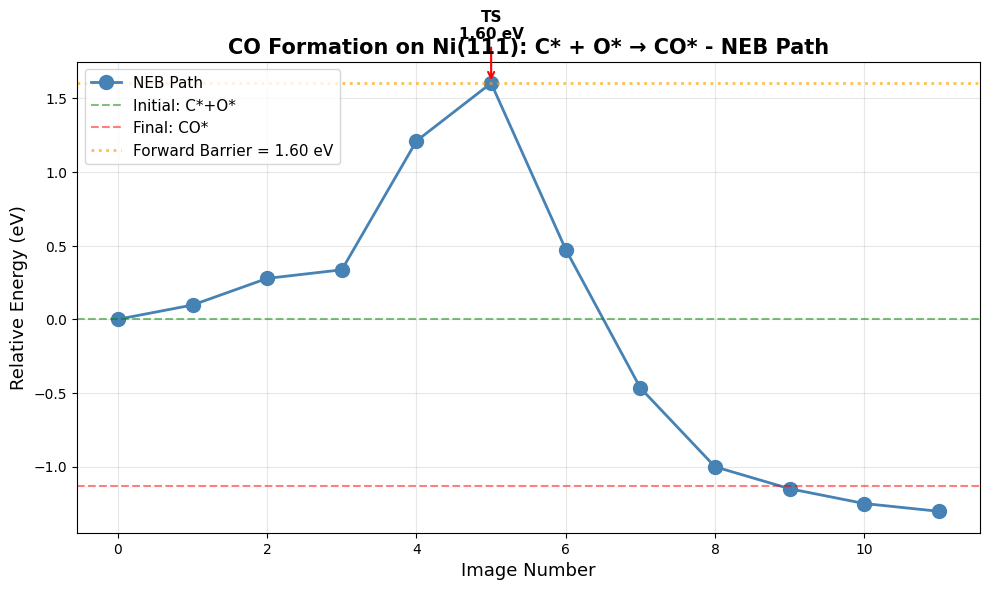
Creating NEB path animation...
→ Saved as neb_path.gif
Visualizing initial state (C* + O*)...
Visualizing transition state...
Visualizing final state (CO*)...
✓ NEB analysis complete!
Explore on Your Own¶
Image convergence: Run with 7 or 9 images. Does the barrier change?
Spring constant: Modify the NEB spring constant. How does this affect convergence?
Alternative paths: Try different initial CO/final C+O configurations. Are there multiple pathways?
Reverse barrier: Calculate E_a(reverse) = E_a(forward) - ΔE. Check Brønsted-Evans-Polanyi relationship.
Diffusion barriers: Compute NEB for C or O diffusion on the surface. How do they compare?
Summary and Best Practices¶
Key Takeaways¶
ML Potentials: UMa-S-1P1 provides ~1000× speedup over DFT with reasonable accuracy
Bulk optimization: Always use the ML-optimized lattice constant for consistency
Surface energies: Linear extrapolation eliminates finite-size effects
Adsorption: Test multiple sites; lowest energy may not be intuitive
Coverage: Lateral interactions become significant above ~0.3 ML
Barriers: NEB requires careful setup but yields full reaction pathway
Recommended Workflow for New Systems¶
Optimize Bulk - Determine equilibrium lattice constant
Calculate Surface Energies - Identify stable facets
Wulff Construction - Predict nanoparticle morphology
Low-Coverage Adsorption - Find binding sites and energies
Coverage Study (if coverage-dependent effects are important) - Determine lateral interactions
Reaction Barriers - Calculate activation energies using NEB
Microkinetic Modeling - Predict overall catalytic performance
Accuracy Considerations¶
| Property | Typical Error | When Critical |
|---|---|---|
| Lattice constants | 1-2% | Strain effects, alloys |
| Surface energies | 10-20% | Nanoparticle shapes |
| Adsorption energies | 0.1-0.3 eV | Thermochemistry |
| Barriers | 0.2-0.5 eV | Kinetics, selectivity |
Rule of thumb: Use ML for screening → DFT for validation → Experiment for verification
Further Reading¶
UMA Paper: Wood et al. 2025
OMat24 Paper: Barroso-Luque et al., 2024
OC20 Dataset: Chanussot et al., ACS Catalysis, 2021
ASE Tutorial: https://
wiki .fysik .dtu .dk /ase/
Appendix: Troubleshooting¶
Common Issues¶
Problem: Convergence failures
Solution: Reduce
fmaxto 0.1 initially, tighten laterCheck if system is metastable (try different starting geometry)
Problem: NEB fails to find transition state
Solution: Use more images (9-11) or better initial guess
Try fixed-end NEB first, then climbing image
Problem: Unexpected adsorption energies
Solution: Visualize structures - check for distortions
Compare with multiple sites
Add D3 corrections
Problem: Out of memory
Solution: Reduce system size (smaller supercells)
Use fewer NEB images
Run on HPC with more RAM
Performance Tips¶
Use batching: Relax multiple configurations in parallel
Start with DEBUG_MAX_STEPS=50: Get quick results, refine later
Cache bulk energies: Don’t recalculate reference systems
Trajectory analysis: Monitor optimization progress with ASE GUI
Caveats and Pitfalls¶
1. Task Selection: OMAT vs OC20¶
Critical choice: Which task_name to use?
task_name="omat": Optimized for bulk and clean surface calculationsUse for: Part 1 (bulk), Part 2 (surface energies), Part 3 (Wulff)
Better for structural relaxations without adsorbates
task_name="oc20": Optimized for surface chemistry with adsorbatesUse for: Part 4-6 (all adsorbate calculations)
Trained on Open Catalyst data with adsorbate-surface interactions
Impact: Using wrong task can lead to 0.1-0.3 eV errors in adsorption energies!
2. D3 Dispersion Corrections¶
Multiple decisions required:
Whether to use D3 at all?
Small adsorbates (H, O, N): D3 effect ~0.01-0.05 eV (often negligible)
Large molecules (CO, CO₂, aromatics): D3 effect ~0.1-0.3 eV (important!)
Physisorption: D3 critical (can change binding from repulsive to attractive)
RPBE was originally fit for chemisorption energies without D3 corrections, so adding D3 corrections may actually cause small adsorbates to overbind. However, it probably would be important for larger molecules. It’s relatively uncommon to see RPBE+D3 as a choice in the catalysis literature (compared to PBE+D3, or RPBE, or BEEF-vdW).
Which DFT functional for D3?
This tutorial uses
method="PBE"consistently for the D3 correction. This is often implied when papers say they use a D3 correction, but the results can be different if use the RPBE parameterizations.Original paper used PBE for bulk/surfaces, RPBE for adsorption. It’s not specified what D3 parameterization they used, but it’s likely PBE.
When to apply D3?
End-point correction (used here): Fast, run ML optimization then add D3 energy
During optimization: Slower but more accurate geometries
Impact: Usually <0.05 eV difference, but can be larger for weak interactions
3. Coverage Dependence Challenges¶
Non-linearity at high coverage:
This tutorial assumes linear E_ads(θ) = E₀ + βθ
Reality: Often non-linear, especially near θ = 1 ML. See the plots generated - there is a linear regime for relatively high coverage, and relatively low coverage, but it’s not uniformly linear everywhere. As long as you consistently in one regime or the other a linear assumption is probably ok, but you could get into problems if solving microkinetic models where the coverage of the species in question changes significantly from very low to high.
Why: Phase transitions, adsorbate ordering, surface reconstruction
Solution: Test polynomial fits, look for ordering in visualizations
Low coverage limit:
At θ < 0.1 ML, coverage effects are tiny (<0.01 eV)
Hard to distinguish from numerical noise
Best practice: Focus on 0.25-1.0 ML range for fitting
4. Periodic Boundary Conditions¶
UMa requires PBC=True in all directions!
atoms.set_pbc([True, True, True]) # Always requiredForgetting this causes crashes or wrong energies
Even for “gas phase” molecules in vacuum
5. Gas Phase Reference Energies¶
Tricky cases:
H₂(g): UMa handles well (used in this tutorial)
H(g): May not be reliable (use H₂/2 instead)
CO(g), O₂(g): Usually okay, but check against DFT
Radicals: Often problematic
Best practice: Always use stable molecules as references (H₂, not H; H₂O, not OH)
6. Spin Polarization¶
Key limitation: OC20/UMa does not include spin!
Paper used spin-polarized DFT
Impact: Usually small (0.05-0.1 eV)
Larger for:
Magnetic metals (Fe, Co, Ni)
Open-shell adsorbates (O*, OH*)
Reaction barriers with radicals
7. Constraint Philosophy¶
Clean slabs (Part 2): No constraints (both surfaces relax)
Best for surface energy calculations
More physical for symmetric slabs
Adsorbate slabs (Part 4-6): Bottom layers fixed
Faster convergence
Prevents adsorbate-induced reconstruction
Standard practice in surface chemistry
Fairchem helper functions: Automatically apply sensible constraints
Trust their heuristics unless you have good reason not to
Check
atoms.constraintsto see what was applied
8. Complex Surface Structures¶
This tutorial uses low-index facets (111, 100, 110, 211)
Well-defined, symmetric
Easy to generate and analyze
Real catalysts have:
Steps, kinks, grain boundaries
Support interfaces
Defects and vacancies
Challenge: Harder to generate, more configurations to test
9. Slab Thickness and Vacuum¶
Convergence tests critical but expensive:
This tutorial uses “reasonable” values (4-8 layers, 10 Å vacuum)
Always check convergence for new systems
Especially important for:
Metals with long electron screening (Au, Ag)
Charged adsorbates
Strong adsorbate-induced reconstruction
10. NEB Convergence¶
Most computationally expensive part:
May need 7-11 images (not just 5)
Initial guess matters a lot
Can get stuck in local minima
Tricks:
Use dimer method to find better TS guess (as shown in Part 6)
Start with coarse convergence (fmax=0.2), refine later
Visualize the path - does it make chemical sense?
Try different spring constants (0.1-1.0 eV/Å)
11. Lattice Constant Source¶
Consistency is key:
Use ML-optimized lattice constant throughout (as done here)
Don’t mix: ML lattice + DFT surface energies = inconsistent
Alternative: Use experimental lattice constant for everything
12. Adsorbate Placement¶
Multiple local minima:
Surface chemistry is not convex!
Always test multiple adsorption sites
Fairchem helpers generate ~5 configurations in this tutorial, but you may need more to search many modes. You can already try methods like minima hopping or other global optimization methods to sample more configurations.
For complex adsorbates:
Test different orientations
May need 10-20 configurations
Consider genetic algorithms or basin hopping
- Kreitz, B., Wehinger, G. D., Goldsmith, C. F., & Turek, T. (2021). Microkinetic Modeling of the CO2 Desorption from Supported Multifaceted Ni Catalysts. The Journal of Physical Chemistry C, 125(5), 2984–3000. 10.1021/acs.jpcc.0c09985
- Chanussot, L., Das, A., Goyal, S., Lavril, T., Shuaibi, M., Riviere, M., Tran, K., Heras-Domingo, J., Ho, C., Hu, W., Palizhati, A., Sriram, A., Wood, B., Yoon, J., Parikh, D., Zitnick, C. L., & Ulissi, Z. (2021). Open Catalyst 2020 (OC20) Dataset and Community Challenges. ACS Catalysis, 11(10), 6059–6072. 10.1021/acscatal.0c04525